Just as solute molecules will passively diffuse down their concentration gradients, water molecules will also move from areas of high concentration to areas of low concentration to equalize the concentration of water inside and outside the cell. The diffusion of water across a membrane is a special type of passive transport called osmosis (FIGURE 3-19). Just as solute molecules may diffuse across a plasma membrane, molecules of water also move across the membrane, equalizing the water concentration inside and outside the cell. Although some water can pass through the lipid bilayer, the hydrophobic region severely limits this flow. Most of the rapid movement of water in and out of cells occurs through “water channels,” called aquaporins, which are transmembrane proteins with hydrophilic channels through which the water molecules pass in single file.
Osmosis can have some dramatic effects on cells. As we saw above, many molecules just can’t move across a cell membrane. But while the molecule can’t move out of the cell and down its concentration gradient, water can move into the cell down its concentration gradient. And as water diffuses into the cell, the cell will get larger.
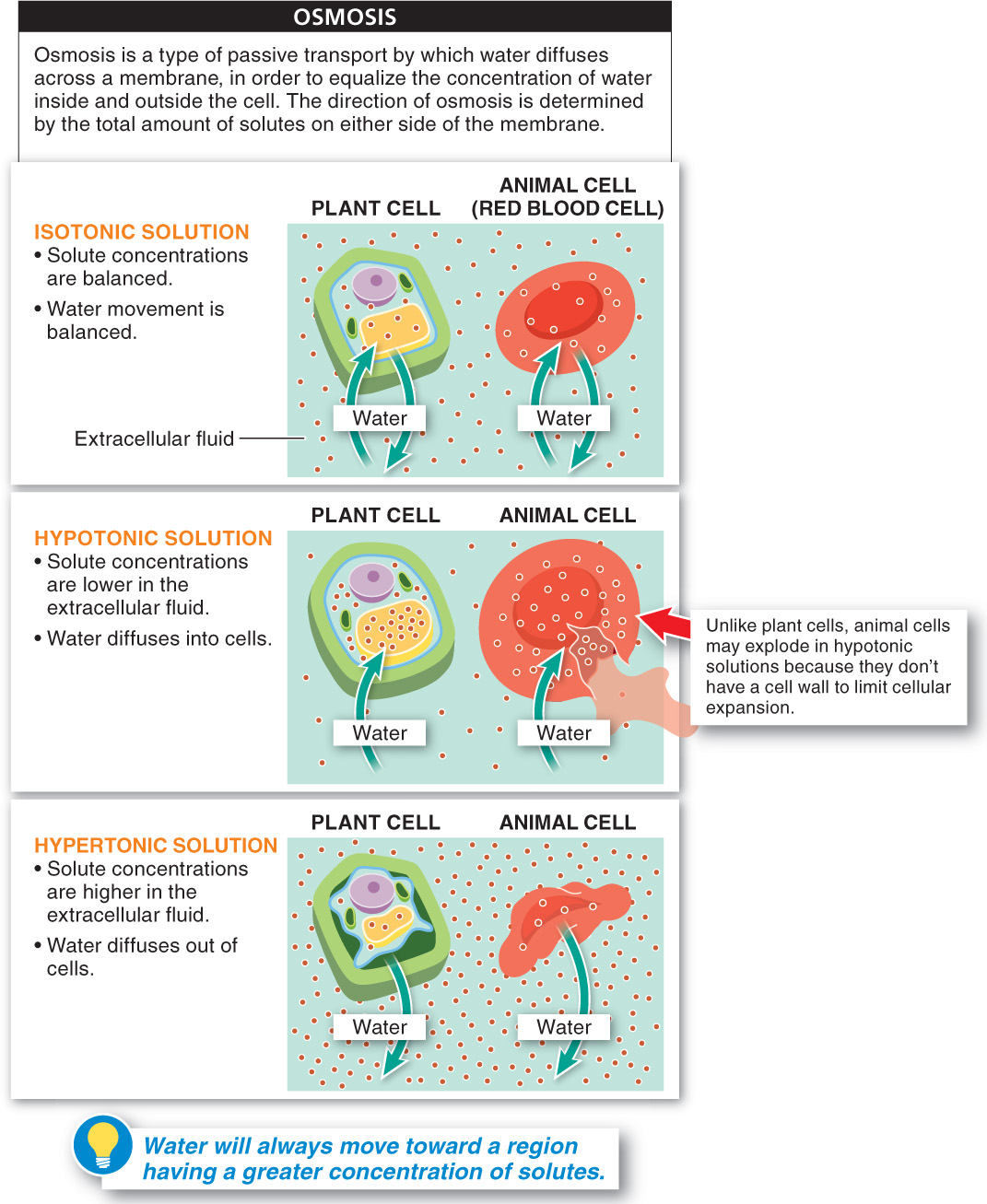
When a cell is in a fluid environment (referred to as a solution), the amount of dissolved substances (solutes) in that solution may be (1) equal to, (2) less than, or (3) greater than the concentration of dissolved substances in the cell. This relationship between the concentrations of solutes inside the cell and solutes outside the cell is referred to as tonicity (see Figure 3-19).
- 1. If the concentration of solutes outside the cell is equal to the concentration inside the cell, the outside solution is isotonic. Water still moves between the solution and the cell, but because it moves at the same rate in both directions, there is no net change in the amount of water inside versus outside the cell
- 2. If the concentration of solutes outside the cell is lower than the concentration inside the cell, the outside solution is hypotonic. In this situation, if the cell membrane is not permeable to the solutes, more water will move into the cell than out of it, and the cell will swell.
- 3. If the concentration of solutes outside the cell is higher than the concentration inside the cell, the outside solution is hypertonic. In this situation, if the cell membrane is not permeable to the solutes, more water will move out of the cell than into it, and the cell will shrivel.
Drinking seawater can be deadly. Why?
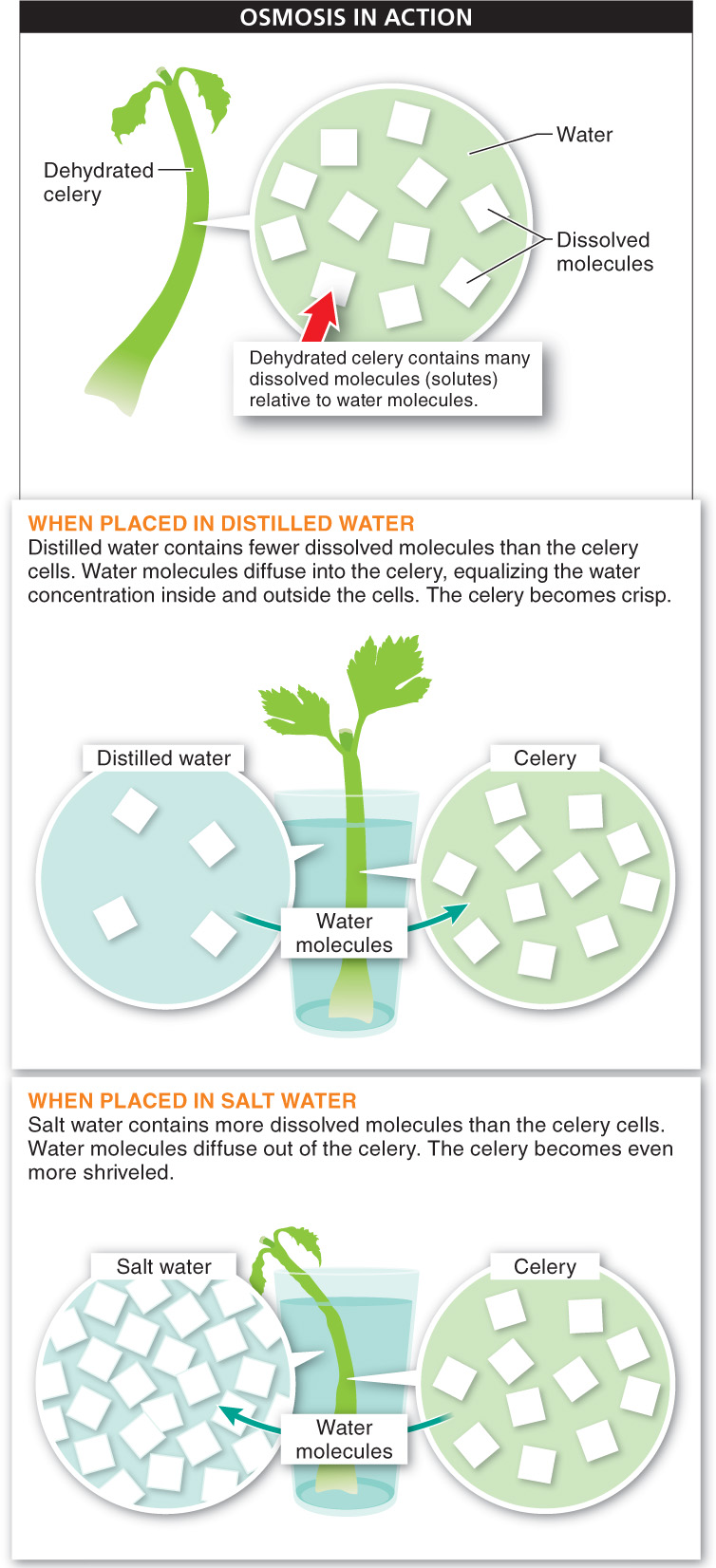
You can see osmosis in action in your own kitchen (FIGURE 3-20). Take a stalk of celery and leave it on the counter for a couple of hours. As water evaporates from the cells of the celery stalk, it will shrink and become limp.
105
You can make it crisp again by placing it in a solution of distilled water. Why distilled water? Because it contains very few dissolved molecules—
How do laxatives relieve constipation?
A more practical use of osmosis is seen with the laxative Milk of Magnesia. This product contains magnesium salts, which are poorly absorbed from the digestive tract. As a consequence, after a dose of the laxative, water moves by osmosis from the surrounding cells into the intestines. The water softens the feces in the intestines and increases the fecal volume, thereby relieving constipation.
It’s important to note that the direction of osmosis is determined only by a difference in the total concentration of all the molecules dissolved in the water: it does not matter what the solutes are, only how many molecules of solutes there are. To determine which way the water molecules will move, you need to determine the total amount of “dissolved stuff” on either side of the membrane. The water will move toward the side with the greater concentration of solute. For this reason, even small increases in the salinity of lakes can have disastrous consequences for the organisms living there, from fish to bacteria. Conversely, putting animal cells—
TAKE-HOME MESSAGE 3.9
The diffusion of water across a membrane is a special type of passive transport called osmosis. Water moves from an area with a lower concentration of solutes to an area with a higher concentration of solutes. Water molecules move across the membrane until the concentration of water inside and outside the cell is equalized.
Compare and contrast what would happen to a plant cell versus an animal cell (such as a human red blood cell) when placed in a hypotonic solution, a hypertonic solution, and an isotonic solution.
Placing a plant cell and a red blood cell in a hypotonic solution would cause both cells to gain water. The red blood cell would swell and potentially burst as it filled with water, whereas the plant cell’s cell wall would prevent it from bursting (although, it would experience turgor pressure and swell slightly). When placing these cells in a hypertonic solution, both would lose water to the solution, causing them to shrink/shrivel; the plant cell would shrink only to a point, limited by its cell wall. In an isotonic solution, both types of cells will have water diffusing into and out of them at equal rates, and the cells will neither burst nor shrink.
106