
Every beer brewery, the entire wine industry, and all distilleries of whiskey, vodka, tequila, and other alcoholic beverages owe their existence to microscopic yeast cells scrambling to break down their food for energy under stressful conditions. To better understand how yeast metabolism produces alcohol, it helps to begin by investigating what happens when humans and other animals try to metabolize energy from sugar molecules under some stressful conditions.
If you run or swim as fast as you can, you soon feel a burning sensation in your muscles. Why? Your muscle cells are becoming very acidic. This acid buildup occurs when we demand of our bodies bursts of energy beyond what they can sustain (FIGURE 4-36). (The next-

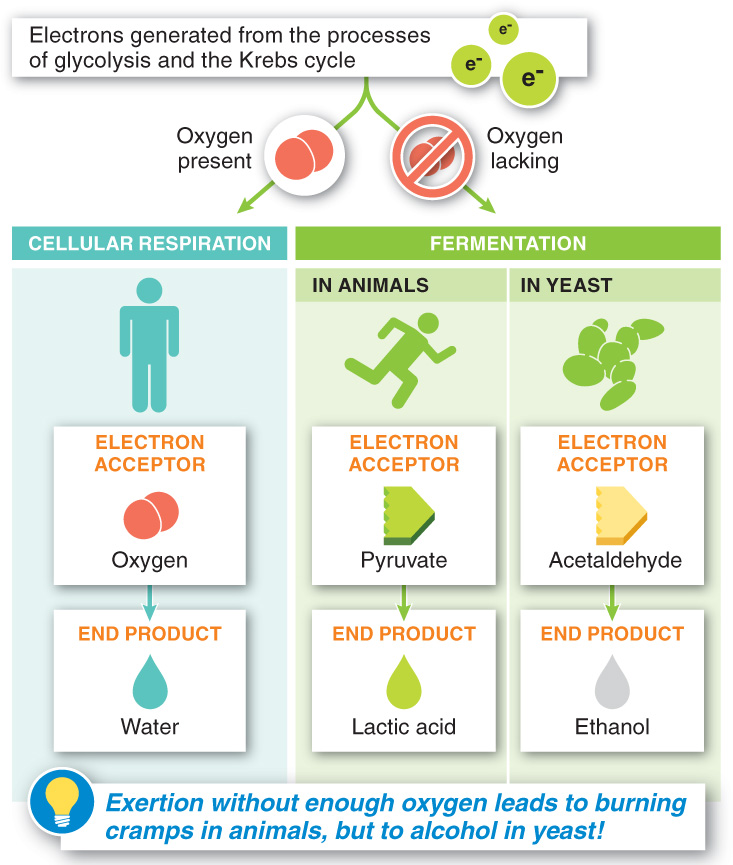
With rapid, strenuous exertion, our bodies soon fall behind in delivering oxygen from the lungs to the bloodstream to the cells and finally to the mitochondria. Oxygen deficiency then limits the rate at which the mitochondria can break down fuel and produce ATP (FIGURE 4-37). This slowdown occurs because the electron transport chain requires oxygen as the final acceptor of all the electrons generated during glycolysis and the Krebs cycle. If oxygen is in short supply, the electrons from NADH (and FADH2) have nowhere to go. Consequently, the regeneration of NAD+ (and FAD) in the electron transport chain is halted, leaving no recipient molecules for the high-
Among animals, there is an acceptor for the NADH electrons in the absence of oxygen: pyruvate, the end product of glycolysis. When pyruvate accepts the electrons, it forms lactic acid (see Figure 4-37). Once the NADH gives up its electrons, NAD+ is regenerated, and glycolysis can continue. But as lactic acid builds up, it causes a burning feeling in our muscles. It’s not ideal, but to escape a predator or to exercise strenuously, the two ATP molecules generated from each glucose molecule during glycolysis—
168
Like humans, yeast normally use oxygen during their breakdown of food. And like humans, they have a backup method when oxygen is not available. But yeast make use of a different electron acceptor, and the resulting reaction leads to the production of all drinking alcohol.
After glycolysis in these single-
Fermentation is the process by which cells obtain energy in the absence of oxygen. It occurs when, following glycolysis, alternative molecules are used as electron acceptors. Interestingly, although ethanol is always the alcohol produced by fermentation, the flavor of the output of fermentation depends on the type of sugar metabolized by the yeast. Fruits, vegetables, and grains all give different results. If the sugar comes from grapes, wine is produced. If the sugar comes from a germinating barley plant, beer is produced. Potatoes, on the other hand, are the sugar source usually used to produce vodka.
Because yeast prefer the more efficient process of aerobic respiration, they produce alcohol only in the absence of oxygen. Fermentation tanks used in producing wine, beer, and other spirits are built to keep oxygen out so that yeast cells are forced to use their backup pathway of fermentation (FIGURE 4-38).

TAKE-HOME MESSAGE 4.17
Oxygen deficiency limits the breakdown of fuel because the electron transport chain requires oxygen as the final acceptor of electrons during the chemical reactions of glycolysis and the Krebs cycle. When oxygen is unavailable, yeast resort to fermentation, in which they use a different electron acceptor, acetaldehyde, and in the process generate ethanol, the alcohol in beer, wine, and spirits.
Compare and contrast fermentation in animals and in yeast.
Fermentation involves the partial breakdown of glucose in the absence of oxygen. Both processes produce energy in the form of ATP without oxygen. This results in the production of lactic acid in animals and the production of ethanol and carbon dioxide in yeast.
169