
Imagine that you are on a long road trip. Your car’s fuel gauge is nearing empty, so you pull off the highway. Instead of driving into a gas station, however, you head to the back of a fast-
Humans can get energy from food. Can machines?
Fast food for your car? Yes! The idea isn’t as far-

It turns out that biofuels, fossil fuels, and the food fuels that supply energy to most living organisms are chemically similar. This fact is not surprising because energy from the sun is the source of the energy stored in the chemical bonds between the atoms in all these fuels. Let’s investigate how fuels provide energy.
When we burn gasoline, long chains of carbon and hydrogen atoms are broken down. As the bonds linking those atoms are broken and molecules with lower-
Animal fats and the oils in many plants—
137
Cars that run on biofuels are more than just a technological trick. The production of biofuels requires only the plant or animal source, sunlight, air, water, and a relatively short amount of time—
Are we at the point yet where all our cars can run on biofuels? Not quite. There are several significant drawbacks to the increased use of biofuels, chief among them the destruction of forests, wetlands, and other ecologically important habitats resulting from the increased use of land to grow these fuels. (And fossil fuel and water are used in the process.) This is why the search for better fuels continues.
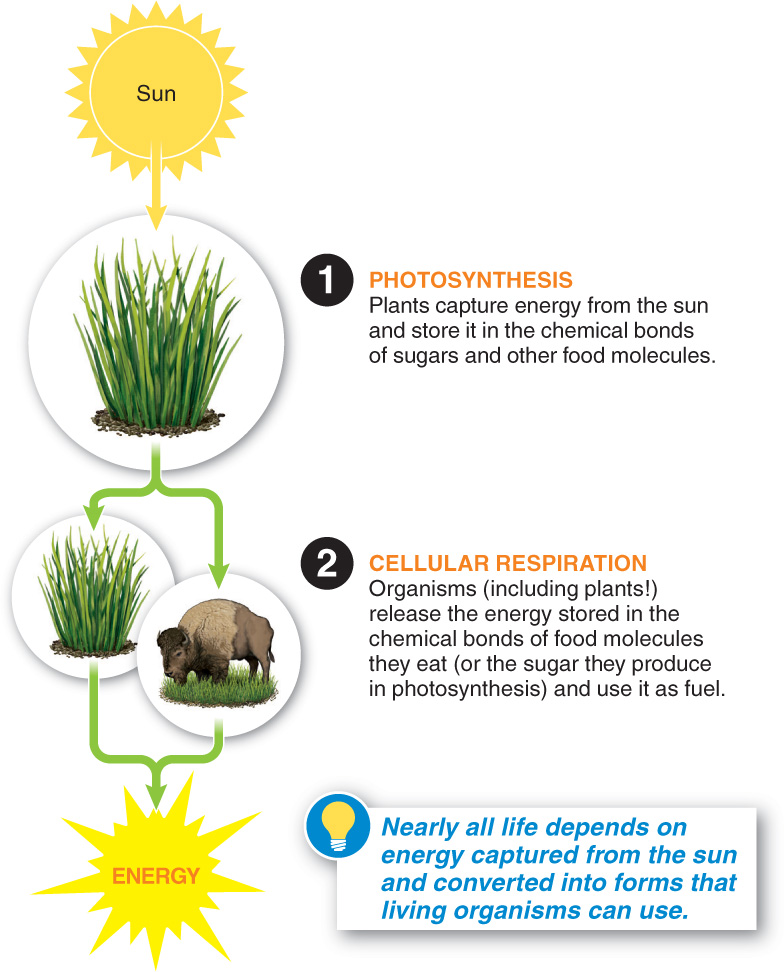
In this chapter we explore how plant, animal, and other living “machines” run on energy stored in chemical bonds. Nearly all life depends on capturing energy from the sun and converting it into forms that living organisms can use. This energy capture and conversion occurs in two important processes that mirror each other: (1) photosynthesis, the process by which plants capture energy from the sun and store it in the chemical bonds of sugars and (2) cellular respiration, the process by which all living organisms release the energy stored in the chemical bonds of food molecules and use it to fuel their lives (FIGURE 4-2). The sun to you in just two steps!
TAKE-HOME MESSAGE 4.1
The sun is the source of the energy that powers most living organisms and other “machines.” The energy from sunlight is stored in the chemical bonds of molecules. When these bonds are broken, energy is released, regardless of whether the bond is in a molecule of food, of a fossil fuel, or of a biofuel such as the oil in which french fries are cooked.
What do fossil fuels, biofuels, and the food we eat all have in common from an energetic and chemical perspective?
Fossil fuels, biofuels, and food all have energy from the sun as the source of the energy stored in the chemical bonds between their atoms. Likewise, all three contain chains of carbon and hydrogen atoms bound together, and breaking these bonds releases the stored energy. If this energy is harnessed, it can be used to do work, such as cellular work or mechanical work.