
In the beginning, there was nothing. Now there is something. That, in a nutshell, describes one of the most important, yet difficult to resolve, questions in science: how did life on earth begin? We open our investigation by describing a few things that we do know and exploring how they help us speculate about the things we do not yet know. First, let’s give a basic definition of what we mean by “life.” Life is defined by the ability to replicate and by the presence of some sort of metabolic activity (the chemical processes by which molecules are acquired and used and energy is transformed in controlled reactions). In the next section we expand on this definition and explore the transition from non-
Earth formed about 4.5 billion years ago from clouds of dust and gases, and as it very gradually cooled, a crust formed at the surface and condensing water formed the oceans. This probably took several hundred million years. The oldest rocks, found in Canada, are about 3.8 billion years old. And the earliest life forms appeared not long after these first rocks formed: fossilized bacteria-
From that initial point in earth’s formation, tremendous biodiversity arose—
How did these first organisms arise? Some have suggested that life may have originated elsewhere in the universe and traveled to earth, possibly on a meteor. It is hotly debated, however, whether microbes could even survive the multi-
407
Phase 1: The formation of small molecules containing carbon and hydrogen. The conditions on earth around the time of the origin of life were very different from those of today. In particular, chemical analyses of old rocks reveal that no oxygen gas was present. The atmosphere included large amounts of carbon dioxide, nitrogen, methane, ammonia, hydrogen, and hydrogen sulfide. Most of these molecules were produced by volcanic eruptions. It was this environment that probably served as the cradle of life, or what Darwin called the “warm little pond” (FIGURE 10-1).
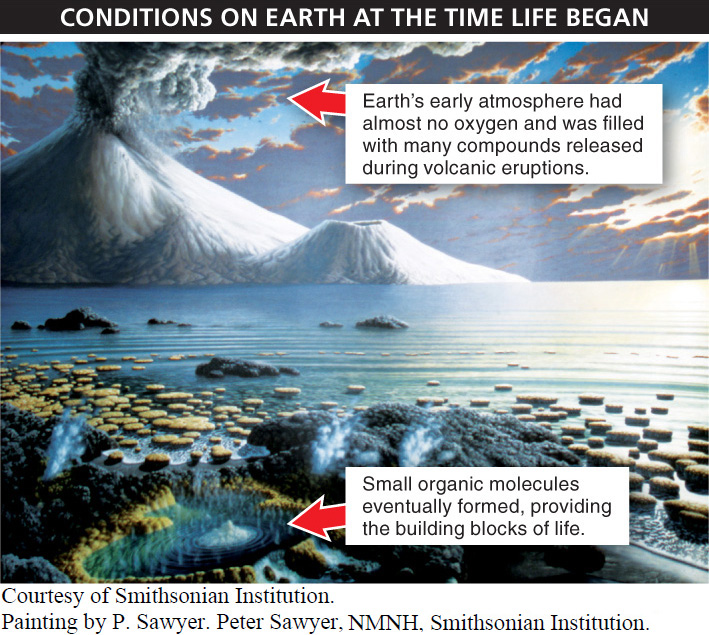
Critical to the origin of life was the formation of small molecules containing carbon and hydrogen. As we saw in Chapter 2, carbon readily bonds with hydrogen and with other carbon atoms, creating molecules with a huge variety of forms that interact with each other in reactions that would eventually become the processes of life. There are several plausible scenarios for how these small organic molecules might have formed. The most likely comes from some simple but revealing four-
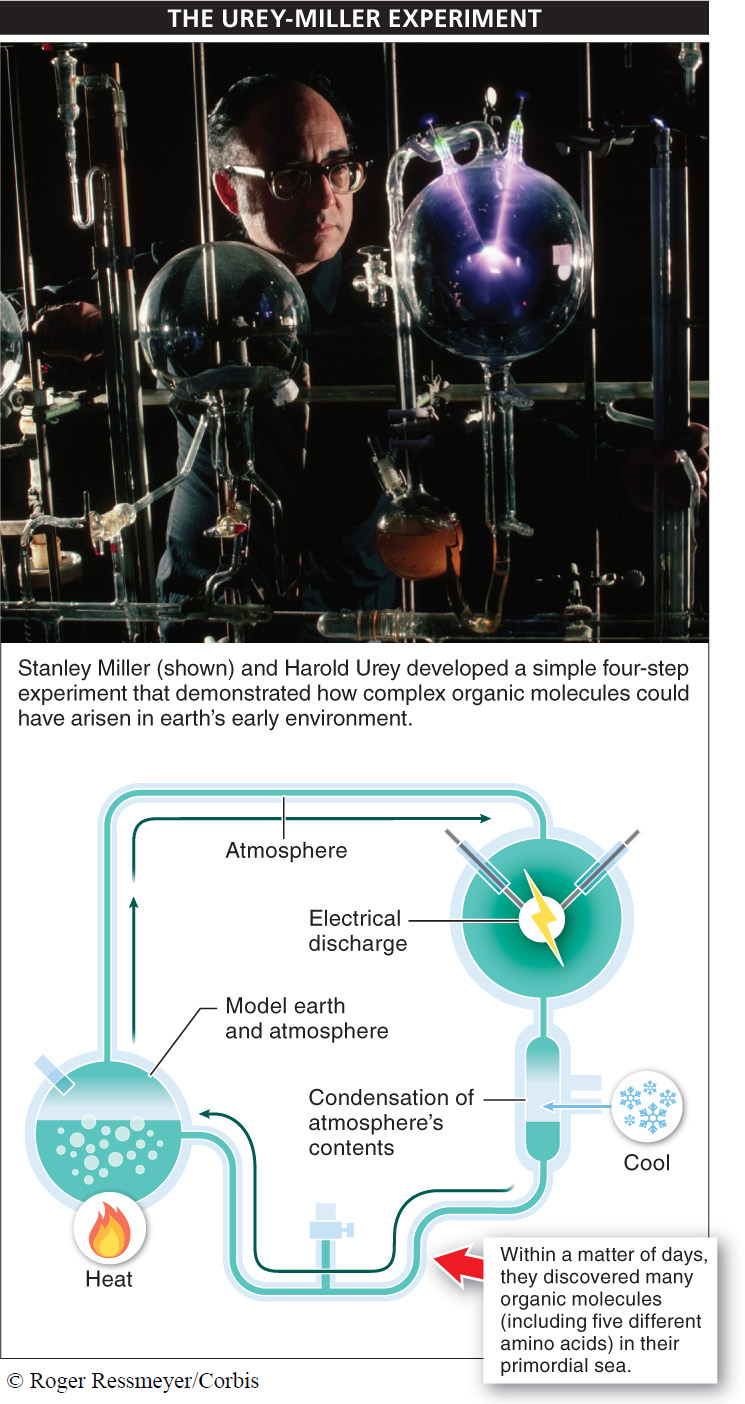
- 1. They created a model of the “warm little pond” and their best estimate of earth’s early atmosphere: a flask of water with H2, CH4 (methane), and NH3 (ammonia).
- 2. They subjected this mini-
world to sparks, to simulate lightning. - 3. They cooled the atmosphere so that any compounds formed in it would rain back down into the water.
- 4. They waited, and then they examined the contents of the water to see what happened.
They didn’t have to wait long to get exciting results. Within a matter of days—
TAKE-HOME MESSAGE 10.1
Under conditions similar to those thought to exist on early earth, small organic molecules can form, and these molecules have some chemical properties of life.
What types of organic molecules were formed in the Urey-
Urey and Miller discovered numerous organic molecules, including five, different amino acids, within a matter of days of running their four-step experiment. Later analysis revealed that all 20 amino acids were produced during their experiments.
408