
Chapter 2. Chapter 2: Chemistry
Review & Rehearse

Instructions
Review the visual summaries and answer the essay questions below.
Make sure to enter a brief response that completely answers each question and explains your reasoning. When you click "Submit," you will be provided instant feedback, allowing you to check if your response is correct.
(This activity contains 20 total essay questions. Each new question will be revealed once you complete the preceding question.)
1.

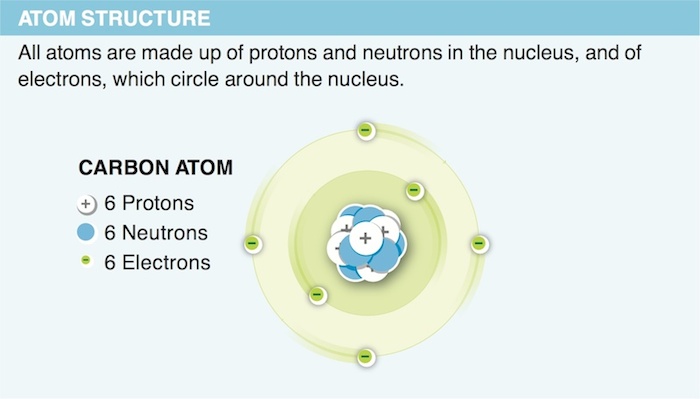
1. When we consider the mass of an atom, why do we ignore the weight of the electrons?
2.
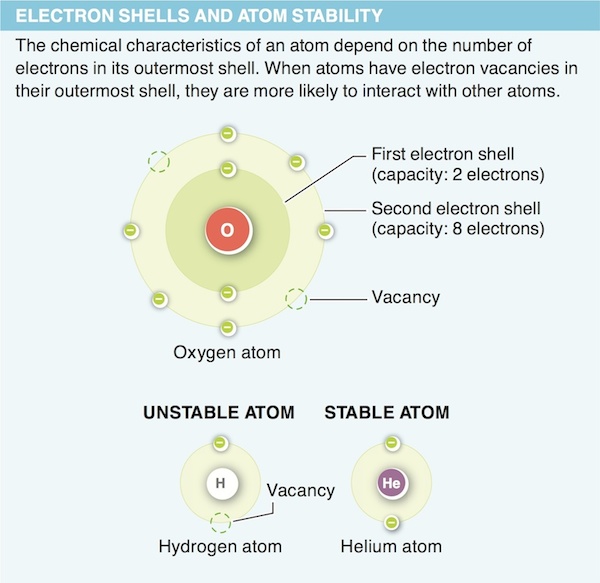
2. Why are atoms with complete outer shells not likely to bond with another atom?
3.
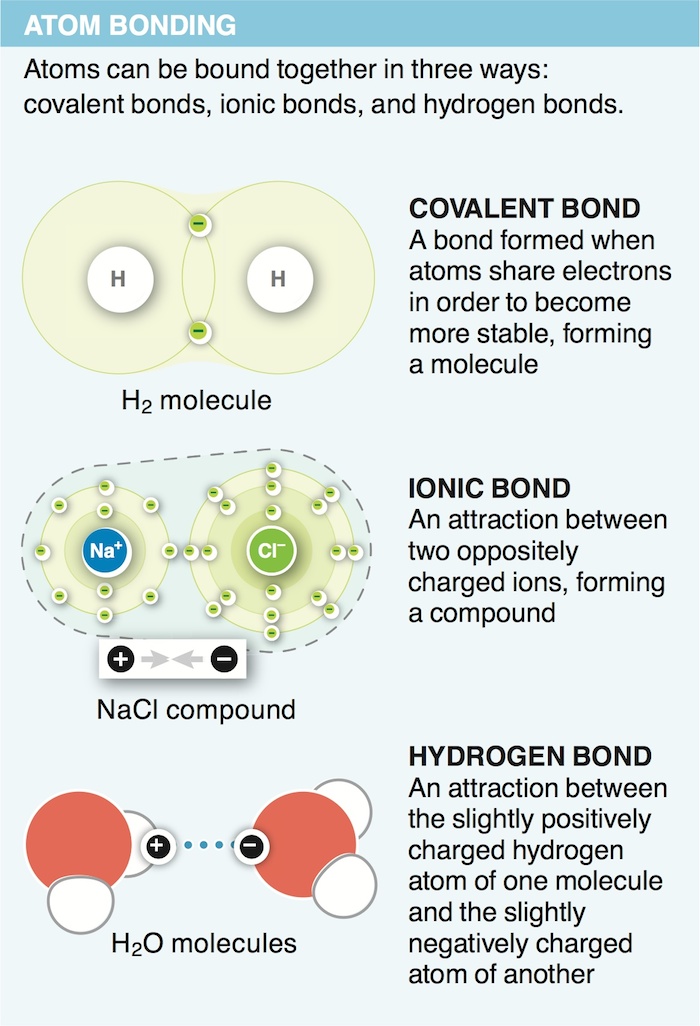
3. Why is a hydrogen atom bonded to another hydrogen atom (to make an H2 molecule) more stable than a hydrogen atom on its own?
4.
4. Why are hydrogen bonds so much weaker than covalent or ionic bonds?
5.

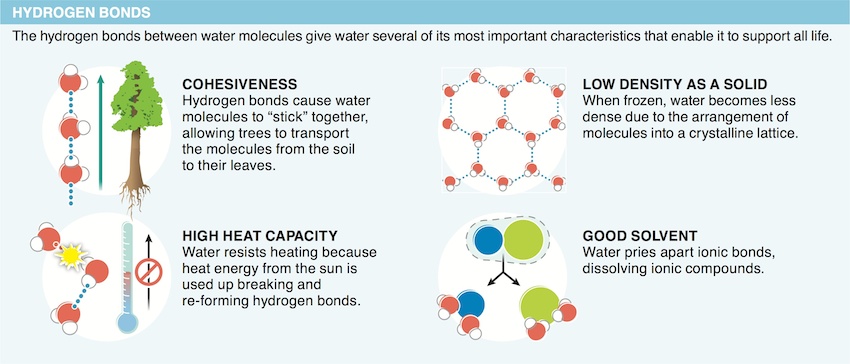
5. Why is water such a good solvent?
6.

6. Why does vomit taste so sour?
7.

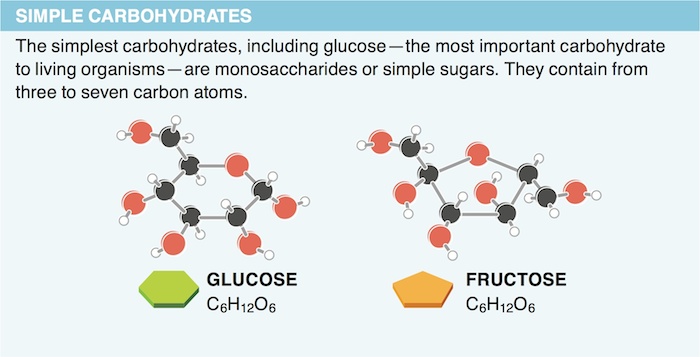
7. Why do carbohydrates function so well as fuel for living organisms?
8.
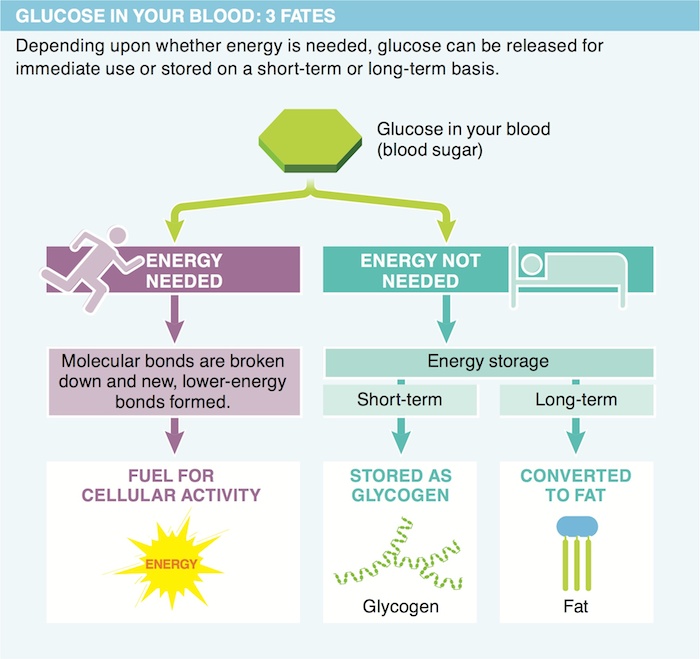
8. Describe the three possible “fates” of glucose in the body.
9.
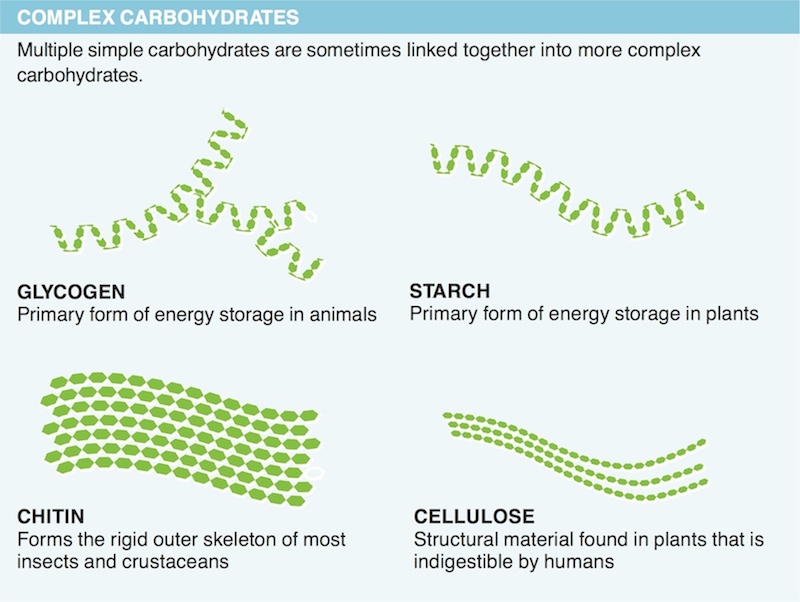
9. Why is it that if you take a piece of potato and leave it on your tongue for a while it starts to taste sweet?
10.
10. Why does cellulose pass through the digestive system unused?
11.

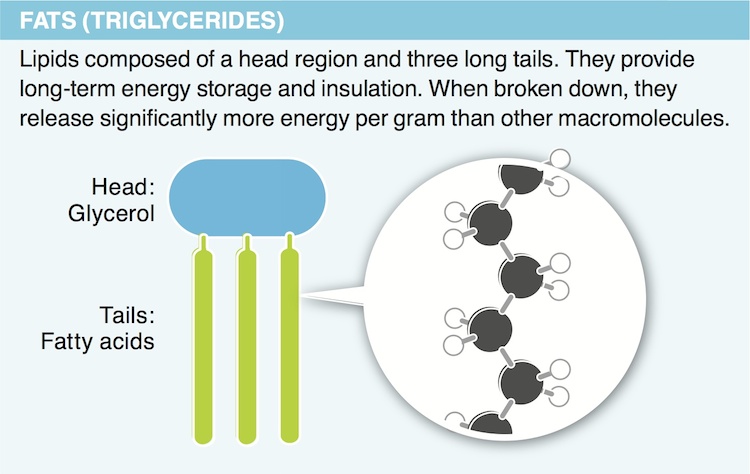
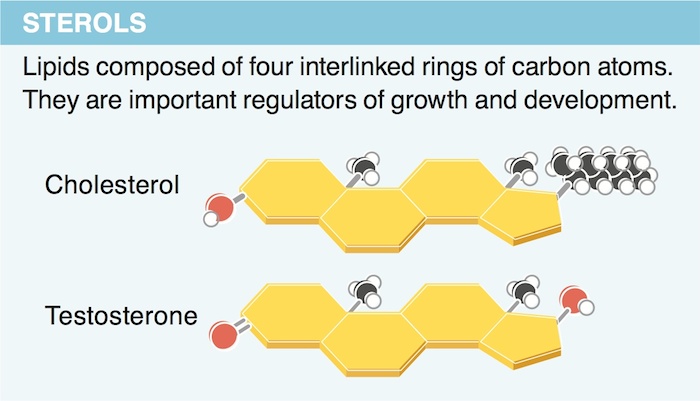
11. Why do lipids contain so much more stored energy than carbohydrates?
12.
12. Why do humans generally have a preference for fats in their diets?
13.
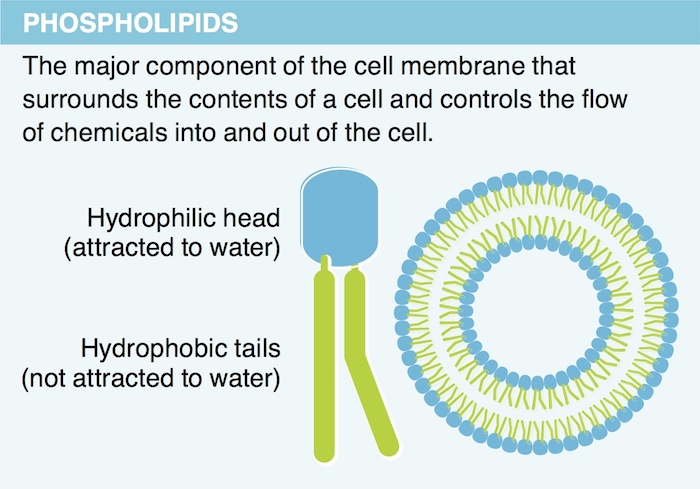
13. Which lipid is an important component of most cell membranes?
14.

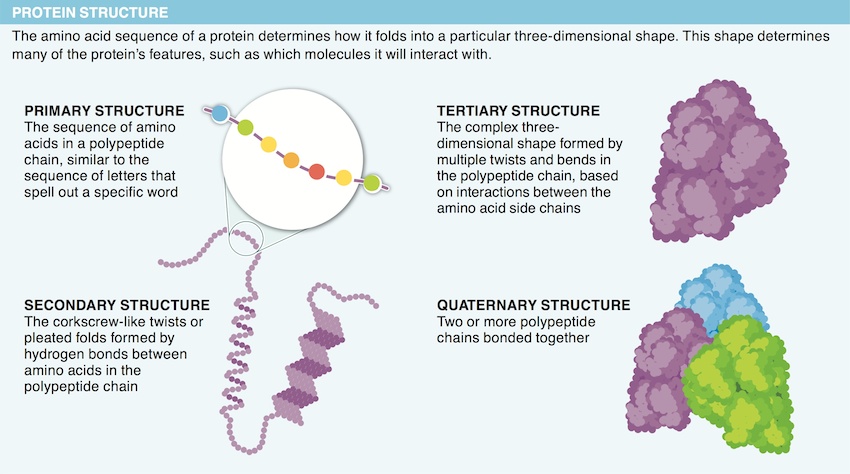
14. Describe two functions proteins serve in the body.
15.
15. What does it mean to say that an amino acid is an “essential amino acid”?
16.
16. How does the amino acid sequence of a protein impact its function?
17.
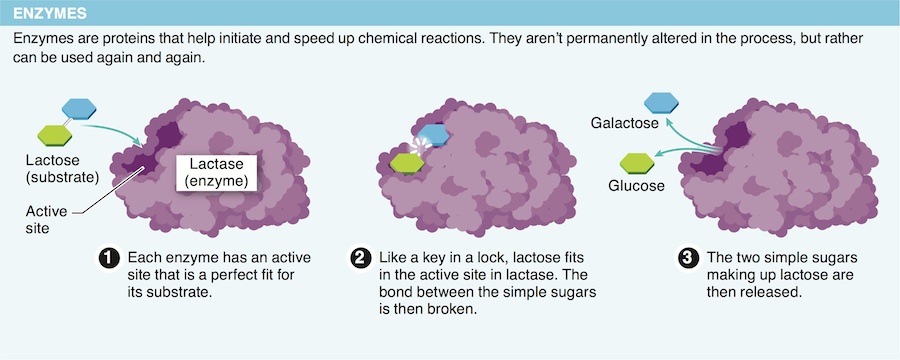
17. Why is protein shape so important for enzymes?
18.

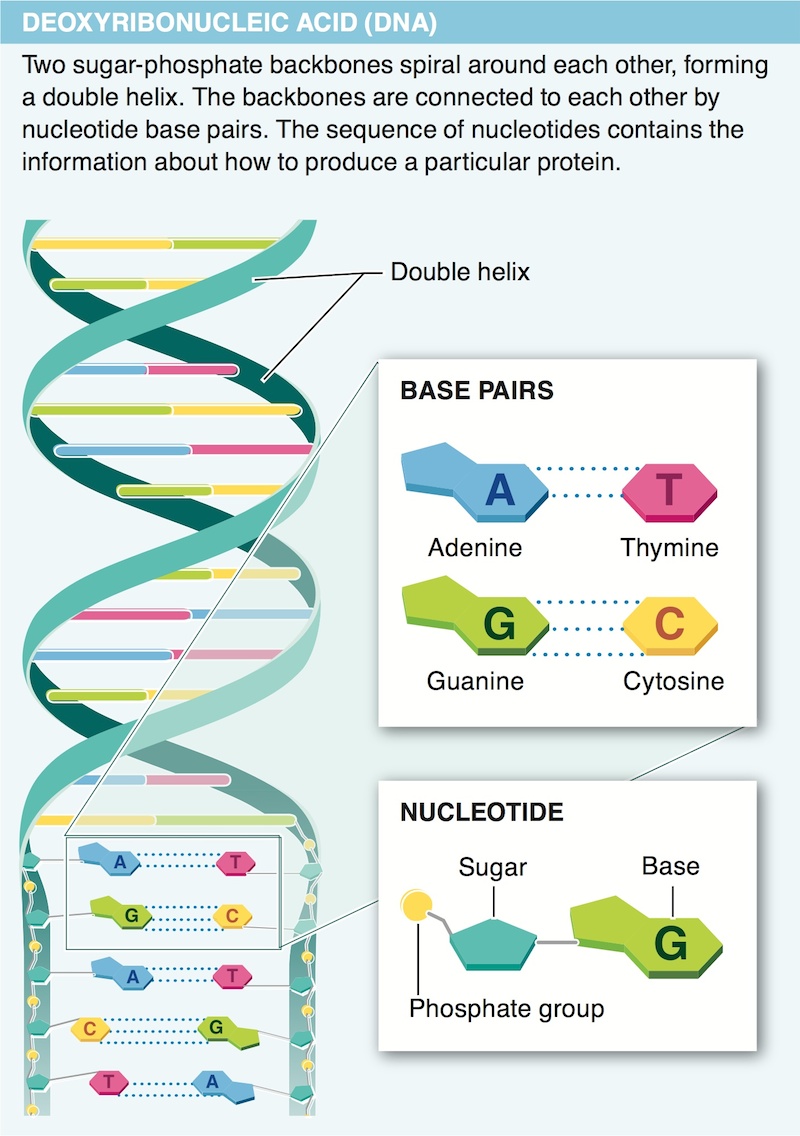
18. How is information stored in the sequence of bases of a nucleic acid?
19.
19. What determines the information in a segment of DNA? Why do researchers working on the Human Genome Project describe only one sequence of nucleotides when presenting a DNA sequence?
20.
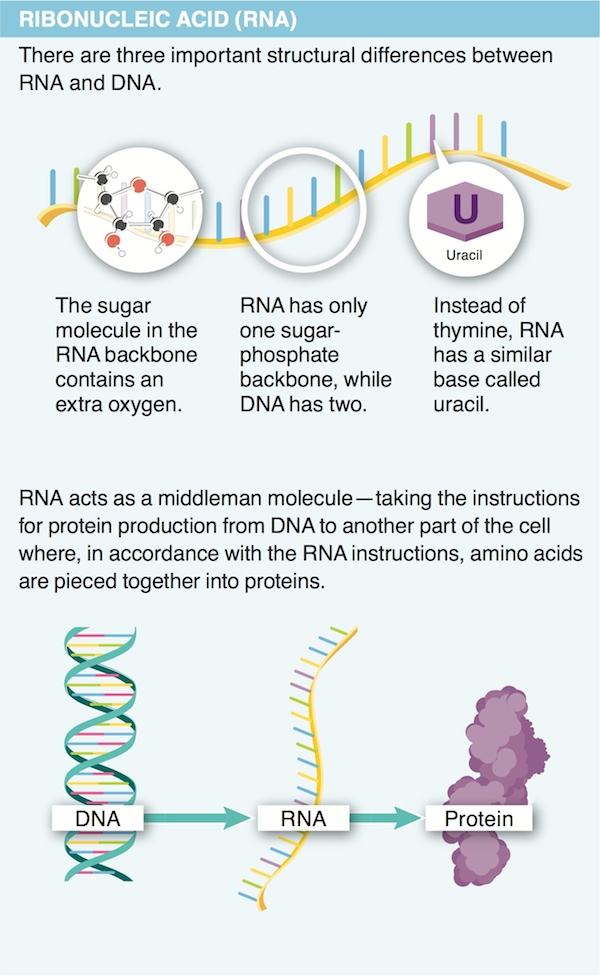
20. Describe the two ways in which RNA differs from DNA.
Activity results are being submitted...