What is necessary for life? Energy and some essential chemicals top the list. New energy continually comes to earth from the sun, fulfilling the first need. And everything else is already here. The chemicals cycle around and around, using the same pathway taken by energy—
Chemicals cycle through the living and non-
We can get a deeper appreciation of the functioning of ecosystems and the ecological problems that can occur when they are disturbed—
Carbon Carbon is found largely in four compartments on earth: the oceans, the atmosphere, terrestrial organisms, and fossil deposits. Plants and other photosynthetic organisms obtain most of their carbon from the atmosphere, where carbon is in the form of carbon dioxide (CO2). As we saw in Chapter 4, photosynthetic organisms use carbon dioxide in photosynthesis, separating the carbon molecules from CO2 and using them to build sugars and other macromolecules. Carbon then moves through the food chain as organisms eat plants and are themselves eaten (FIGURE 15-16). The oceans contain most of the earth’s carbon. Here, many organisms use dissolved carbon to build shells (which later dissolve back into the water, after the organism dies).
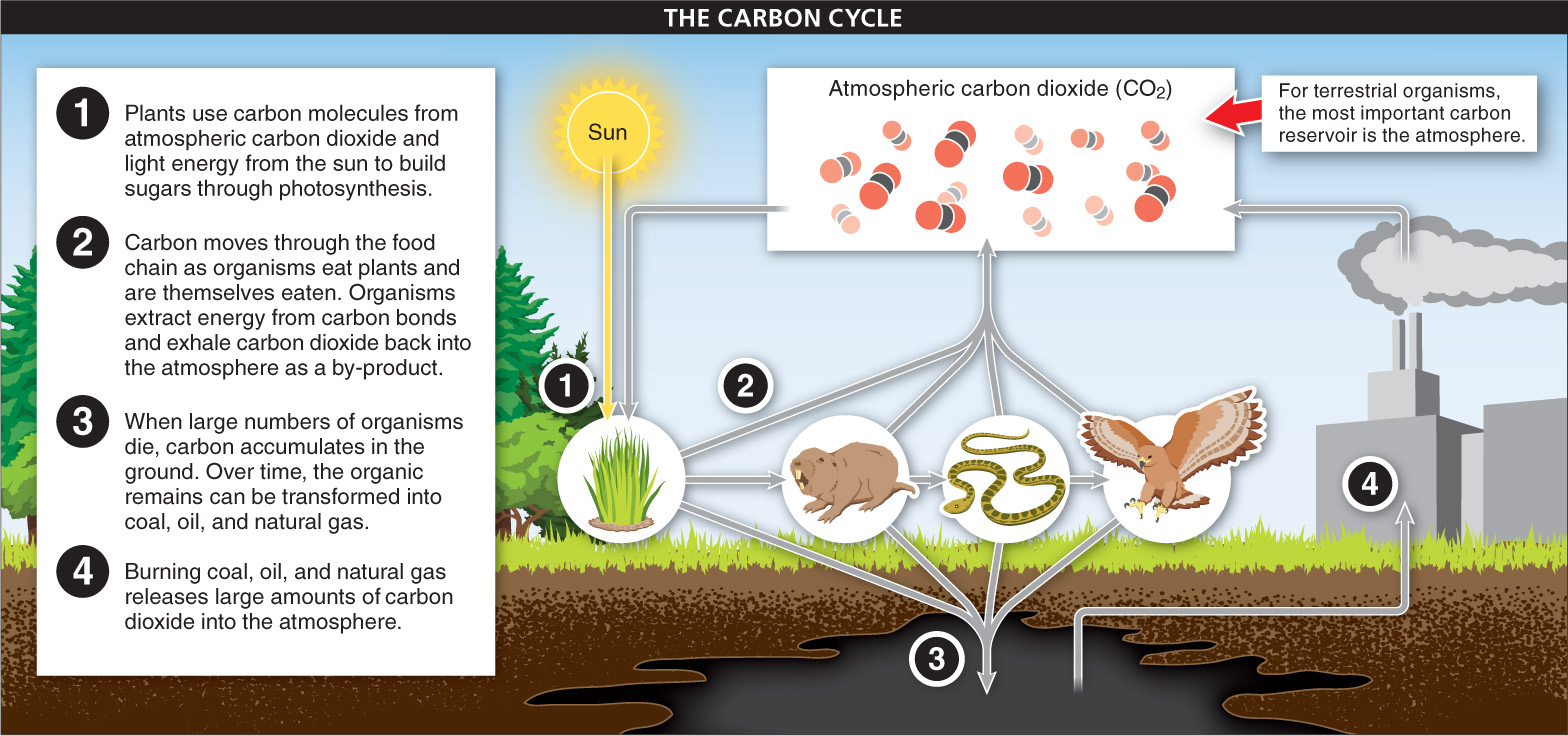
Most carbon returns to its reservoir as a consequence of organisms’ metabolic processes. Organisms extract energy from food by breaking carbon-
625
Why are global CO2 levels rising?
The burning of fossil fuels is adding significantly to the atmospheric carbon reservoir. Fossil fuels are created when large numbers of organisms die and are buried in sediment lacking oxygen. In the absence of oxygen, at high pressures, and after very long periods of time, the organic remains are transformed into coal, oil, and natural gas. Trapped underground or in rock, these sources of carbon played little role in the global carbon cycle until humans in industrialized countries began using fossil fuels to power their technologies. Burning coal, oil, and natural gas releases large amounts of carbon dioxide, thus increasing the average CO2 concentration in the atmosphere—
Global CO2 levels are rising overall, but they also exhibit a sharp rise and fall over the course of each year. Why?
Although, on average, the level of CO2 in the environment is increasing, there is a yearly cycle of ups and downs in the CO2 levels in the northern hemisphere. This is due to fluctuations in the ability of plants to absorb CO2. Many trees lose their leaves each fall and, during the winter months, relatively low rates of photosynthesis lead to low rates of CO2 consumption, causing an annual peak in atmospheric CO2 levels. During the summer, trees have their leaves, sunlight is strong, and photosynthesis (consuming CO2) occurs at much higher levels, causing a drop in the atmospheric CO2 level.
Nitrogen Nitrogen is necessary to build a variety of molecules critical to life, including all amino acids, the components of every protein molecule. The chief reservoir of nitrogen is the atmosphere, but even though more than 78% of the atmosphere is nitrogen gas (N2), for most organisms this nitrogen is completely unusable. The problem is that atmospheric nitrogen consists of two nitrogen atoms bonded tightly together, and these bonds need to be broken to make the nitrogen usable for living organisms. Only through the metabolic magic (chemistry, actually) of some soil-
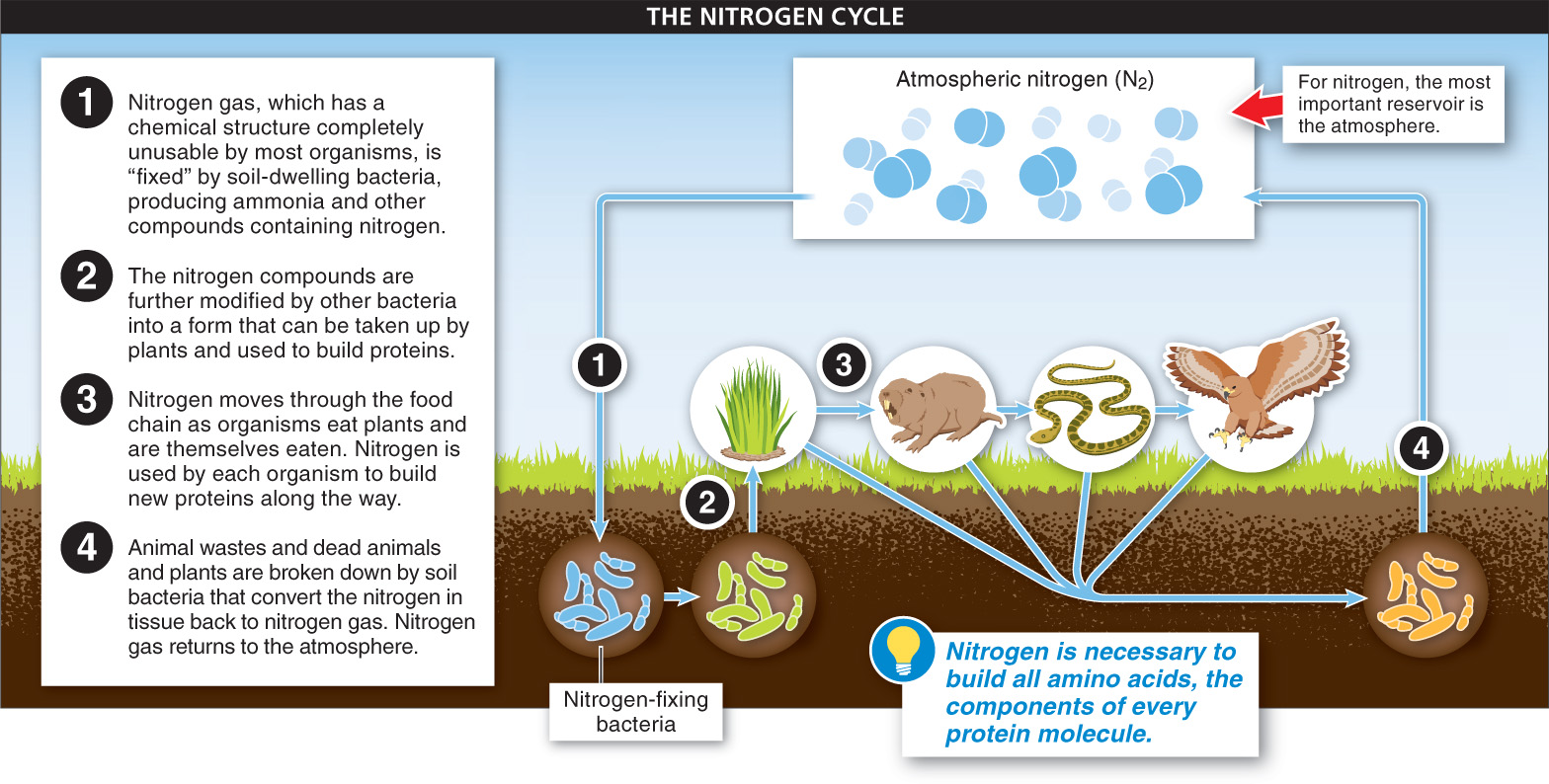
These bacteria chemically convert, or “fix,” nitrogen by attaching it to other atoms, including hydrogen, to produce ammonia and related compounds. These compounds are then further modified by other bacteria into a form that can be taken up by plants and used to build proteins. And once nitrogen is in plant tissues, animals acquire it in the same way they acquire carbon: by eating the plants. Nitrogen returns to the atmosphere when animal wastes and dead animals are broken down by soil bacteria (decomposers) that convert the nitrogen compounds in tissues back to nitrogen gas.
626
The availability of nitrogen is like a bottleneck limiting plant growth. Because it is necessary for the production of every plant protein, and because all nitrogen must first be made usable by bacteria, plant growth is often limited by nitrogen levels in the soil. For this reason, most fertilizers contain nitrogen in a form usable by plants.
Phosphorus Every molecule of ATP and DNA requires phosphorus. But no (or barely any) phosphorus is available in the atmosphere. Instead, soil serves as the chief reservoir. Like nitrogen, phosphorus is chemically converted—
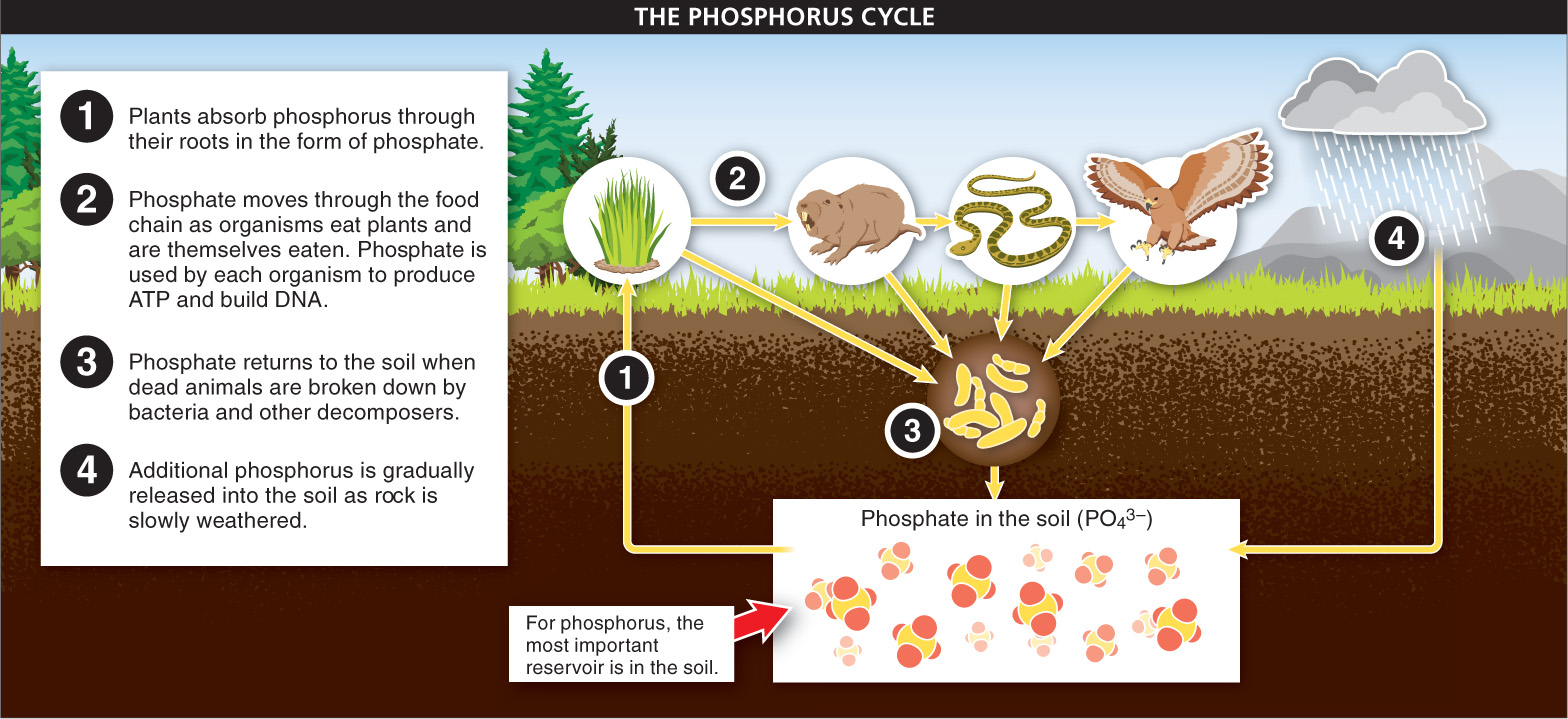
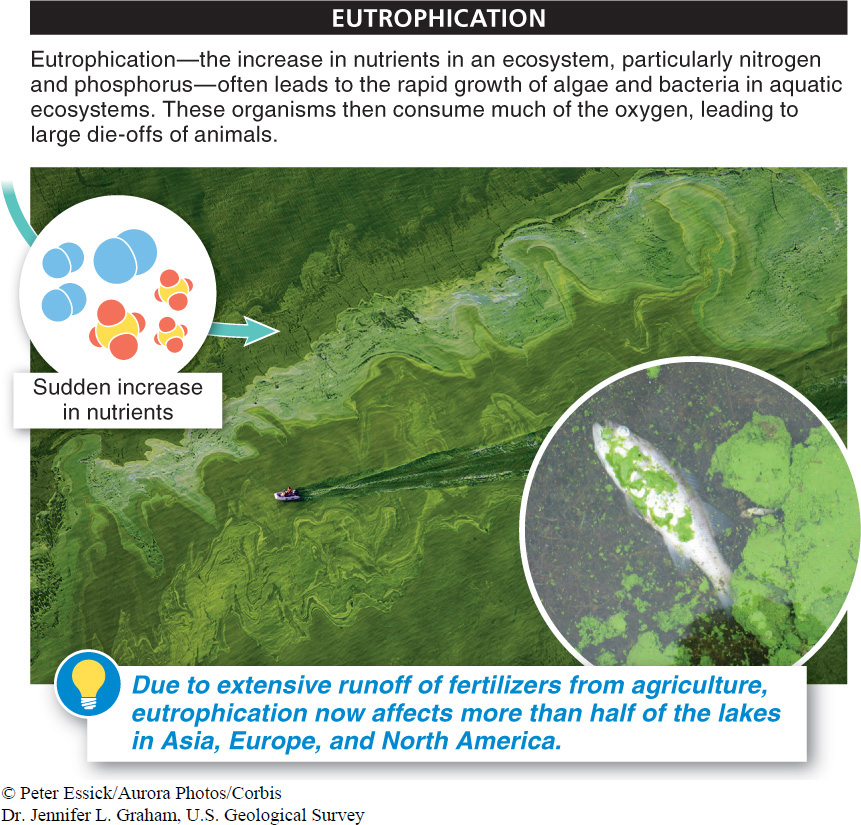
Like nitrogen, phosphorus is often a limiting resource in soils, constraining plant growth. Consequently, fertilizers usually contain large amounts of phosphorus. This is beneficial in the short run, but it can have some disastrous unintended consequences. As more and more phosphorus (and nitrogen) is added to soil, some of it runs off with water and ends up in lakes, ponds, and rivers. It also acts as a fertilizer in these habitats, making spectacular growth possible for algae. But eventually the algae die and sink, creating a huge source of food for bacteria. The bacterial population increases and may use up too much of the dissolved oxygen, causing fish, insects, and many other organisms to suffocate and die.
“An atom of phosphorus ‘X’ had marked time in the limestone ledge since the Paleozoic seas covered the land. Time, to an atom locked in a rock, does not pass. The break came when a bur-
— ALDO LEOPOLD, A Sand County Almanac, 1949
The increase in nutrients, particularly nitrogen and phosphorus, in an ecosystem, is called eutrophication. The consequent rapid growth of algae and bacteria, followed by large-
627
TAKE-HOME MESSAGE 15.8
Chemicals essential to life—
How is ocean water to a thirsty shipwreck victim like nitrogen gas in the atmosphere to most organisms?
Both are abundant and both are unusable. More than 78% of the atmosphere is nitrogen gas; however, for most organisms, nitrogen is only usable after its bonds are broken down in the soil by nitrogen-fixing bacteria.
628