“What goes up must come down.” That’s a saying that applies to molecules just as much as it does to larger objects. The difference is that when molecules come down, they may not be in the same form as when they went up. And in one dangerous instance of this, chemicals that rise into the atmosphere as gases return to earth as acidic precipitations, in the form of fog, sleet, snow, or rain.
We use the term “fossil fuels” to describe oil, natural gas, and coal. Composed largely of carbon and hydrogen, these substances form carbon dioxide (CO2) and water (H2O) when they burn. Although they are referred to as hydrocarbons, fossil fuels are not pure hydrocarbons. In addition to carbon and hydrogen, they also contain substantial quantities of other elements, including sulfur and nitrogen (FIGURE 16-20).

667
When fossil fuels are burned, the gases sulfur dioxide (SO2) and nitrogen dioxide (NO2) are produced. These gases react with water vapor in the atmosphere to produce sulfuric acid (H2SO4) and nitric acid (HNO3), and these acids fall to earth as acid precipitation (FIGURE 16-21).

Why is acid rain an international issue?
In North America, precipitation has an average pH as low as 4.3 in some parts of the Northeast, more than 10 times as acidic as clean rain, which has a pH of about 5.6. (Clean rain is more acidic than pure water because carbon dioxide in the air dissolves in water to form carbonic acid, making the rain slightly acidic.) High concentrations of sulfuric and nitric acids in the atmosphere are to blame, and several factors play a role. The Northeast has more people per square mile than the rest of the United States, and that means there are more houses, factories, and automobiles burning the oil, coal, and gasoline that create acid precipitation (FIGURE 16-22).
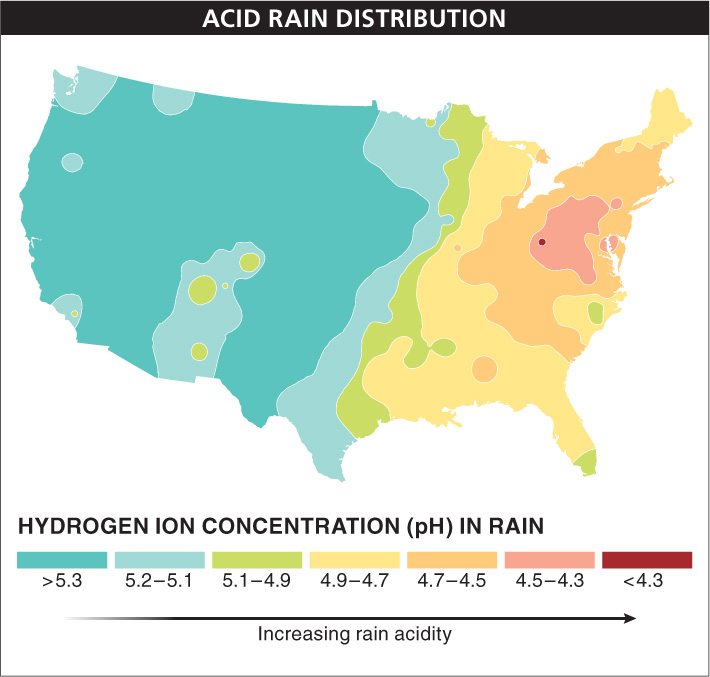
But not all of the pollution that causes acid precipitation is local. In the United States, the Midwest and Southeast have a large number of electric power plants that burn coal, and wind currents carry the sulfur dioxide and nitrogen dioxide produced from this combustion across the Northeast. The precipitation in the western states is not as acidic as that in the Northeast (but it’s still significantly more acidic than clean rain). Much of this region’s acid precipitation comes from sulfur dioxide and nitrogen dioxide from Asia, which are converted to acids as they blow eastward across the Pacific Ocean. Precipitation in Europe and Asia is also acidified by local and distant sources of pollution.
668
Both terrestrial and aquatic organisms are harmed by acid precipitation, and the effects of acid precipitation can be direct or indirect. Direct effects result from contact of living tissues with acidic water, whereas indirect effects are produced by interactions between non-
Dramatic evidence of the direct effects of acid precipitation on vegetation can be found in mountain forests that are often blanketed by fog. When trees are exposed to acid fog, year after year, their leaves are damaged, their rates of photosynthesis decrease, and eventually, many of the trees die (FIGURE 16-23).

The indirect effects of acid precipitation on forests are not as conspicuous as the direct effects, but they are more far-
Lakes and streams are also affected directly and indirectly by acid precipitation, and some lakes in the northeastern United States and in northern Europe have pH values as low as 4.0–
Acid rain is a solvable problem. The deposition of acids from acid rain can be both prevented and cleaned up. The best approach is to prevent or reduce the emissions of sulfur dioxide and nitrogen dioxide. Preventive measures include improving energy efficiency, reducing the use of coal, switching to the use of natural gas, and increasing the use of renewable energy resources, such as wind energy and solar energy. In the United States, many coal-
In the United States, regulations required reductions in sulfur dioxide and nitrogen oxides by 2010 to levels approximately one-
TAKE-HOME MESSAGE 16.9
Burning fossil fuels releases the gases sulfur dioxide and nitrogen dioxide, and these compounds form sulfuric and nitric acids when they combine with water vapor in the atmosphere. Rain, fog, sleet, and snow that contain these acids can be more than 10 times more acidic than clean rain. Acid precipitation kills plants and aquatic animals directly, by contact with living tissues, and indirectly, through changes in soil and water chemistry.
What is the key factor that makes acid rain and acid fog more than just a local phenomenon?
Wind currents can carry sulfur dioxide and nitrogen dioxide far beyond the points of their origin.
669