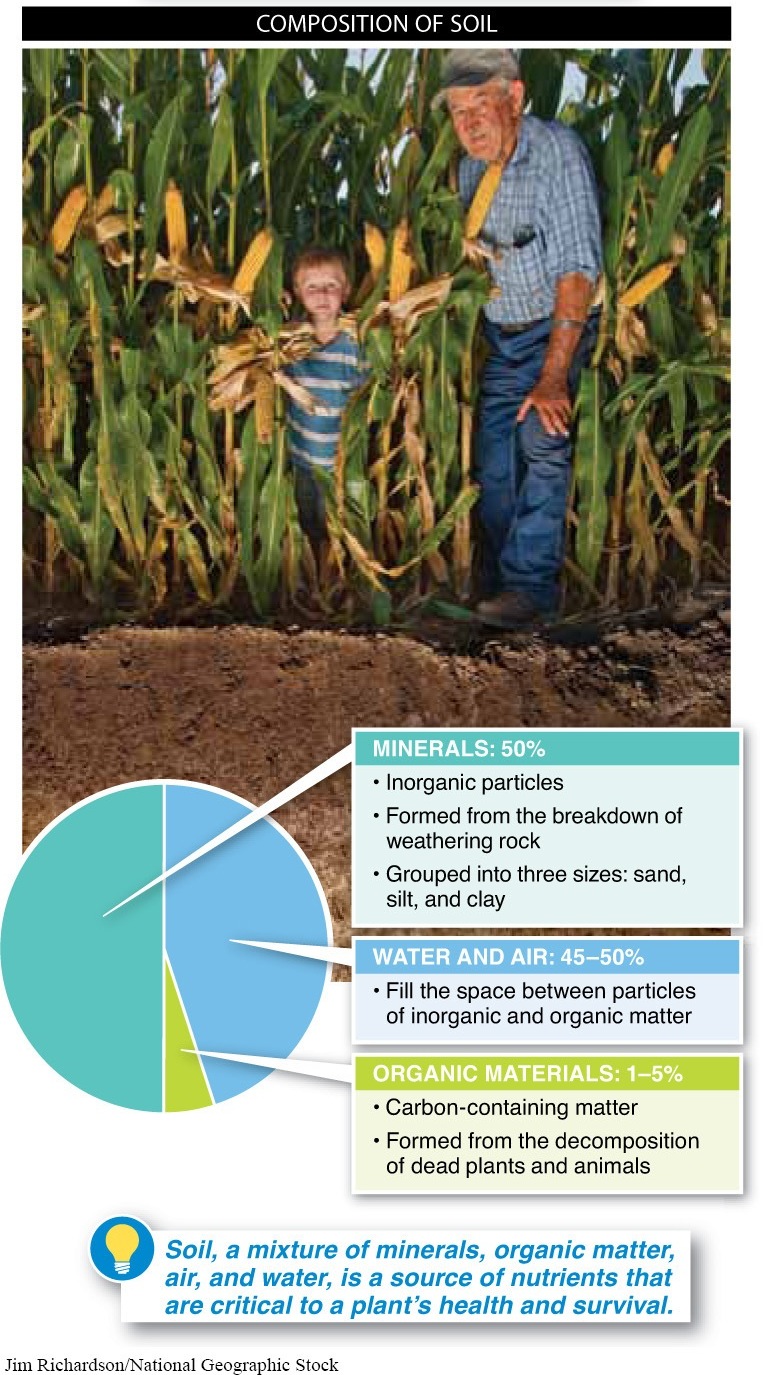
Soil is an almost perpetual source of nutrients that are critical to a plant’s health and survival. But how do the nutrients get there? To answer that, we need to realize that soil is not just a homogeneous mound of dirt. Soil is a complex mixture of four distinct components (FIGURE 17-22): (1) minerals: inorganic particles, usually from the breakdown of rock; (2) organic materials: carbon-
Minerals 705
Minerals About half of the total volume of soil is inorganic materials. These materials come primarily from the weathering of rocks, which decay physically and chemically over time, releasing minerals. The inorganic particles are grouped into three sizes: sand, silt, and clay. As you may know from feeling sand at the beach, there’s plenty of air between the particles, so sand doesn’t hold water very well. That’s why it is so easy to grab handfuls of it and let the grains fall through your fingers. Silt particles are smaller, as you would feel if you grabbed a handful of soil from the bottom of a riverbed. Clay particles, the smallest of all, pack together very densely. Because minerals passing through the soil cling to clay, it is a valuable component of soil; clay holds the essential minerals in place for plants to absorb as needed. However, too much densely packed clay in soil can limit the supply of air available for absorption by plant roots, leading to poor plant growth.
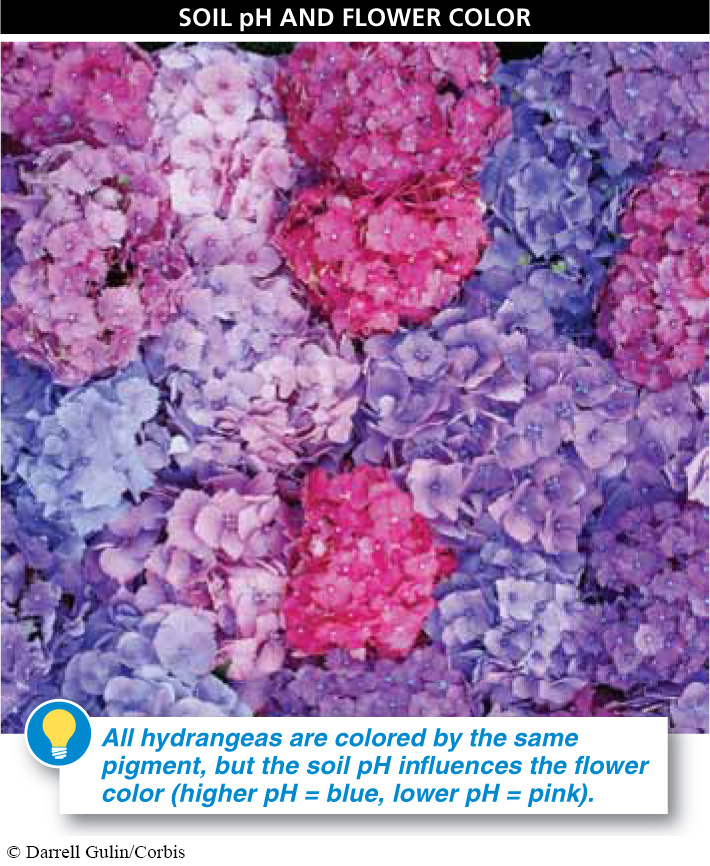
The best soils have about equal amounts of each type of particle. The minerals present in soil determine the pH of the soil and can affect how well plants grow; they can even influence the color of flowers (FIGURE 17-23).
Organic Materials Leaves fall and animals die, but decomposition returns their chemicals to the soil, making reuptake by plant roots possible. Animal droppings are very important to plants, too, serving as highly concentrated sources of nitrogen, perhaps the most important of the essential plant nutrients. Called humus (pronounced HYOO-muhss), all these organic decay products make up about 1% to 5% of the soil (FIGURE 17-24). The humus also absorbs water and nutrients easily and can release them as needed. With too little humus, the soil may be deficient in nutrients. With too much, it may retain too much water.
“I bequeath myself to the dirt
to grow from the grass I love,
If you want me again
look for me under your boot-
—WALT WHITMAN, Leaves of Grass (1855)
706
Most of the decomposition of organic matter is carried out by huge populations of bacteria, fungi, insects, and earthworms that live in soil. Aristotle called earthworms “the intestines of the earth.” Passing soil through their guts as they feed on organic matter, earthworms increase tremendously the amount of nitrogen available for plants.
What is composting? Why is it useful? Is it dangerous?
A very practical application of the decomposition of organic matter can be seen in the process of composting, used to decompose organic wastes—
Does human waste have value as fertilizer?

The growing populations of bacteria in compost generate large amounts of heat—

Water and Air Water and air, the final components of soil, fill the spaces between the particles of inorganic and organic matter. They account for about half of the total volume of soil.
TAKE-HOME MESSAGE 17.9
Soil is a mixture of minerals, organic materials, air, and water that serves as an almost perpetual source of nutrients critical to a plant’s health and survival.
Why is organic material an important part of soil?
Decayed organic material, called humus, absorbs and releases water, and also releases many nutrients.
707