Reading these words requires carbohydrates in your body, obtained from your diet, to fuel your brain—
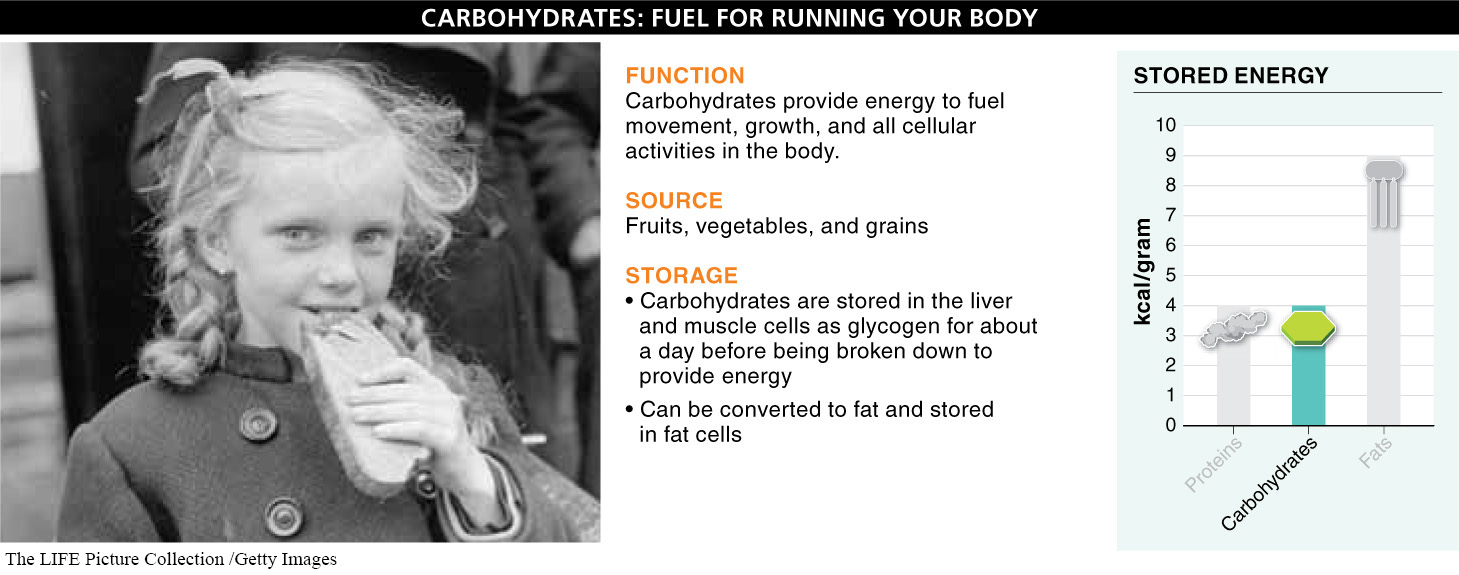
Carbohydrates: Fuel for Living Machines What are carbohydrates, and what is their role in the diet? Carbohydrates are the primary fuel on which animal bodies run. In humans, nearly all of the energy used by our brain every day comes from the simple carbohydrate glucose. As we saw in Chapter 2, all carbohydrates are made primarily from carbon, hydrogen, and oxygen. When the bonds between these atoms are broken, energy is released that can be captured by the body and used to fuel movement, growth, and all the other cellular activities that require energy.
882
We get the majority of our dietary carbohydrates from fruits, vegetables, and grains (FIGURE 22-9). And, as with proteins, the breakdown of carbohydrates for energy generates 4 kilocalories per gram.
Are all carbohydrates the same, or do they vary in important ways? Although, structurally, all carbohydrates are variations on a simple theme—
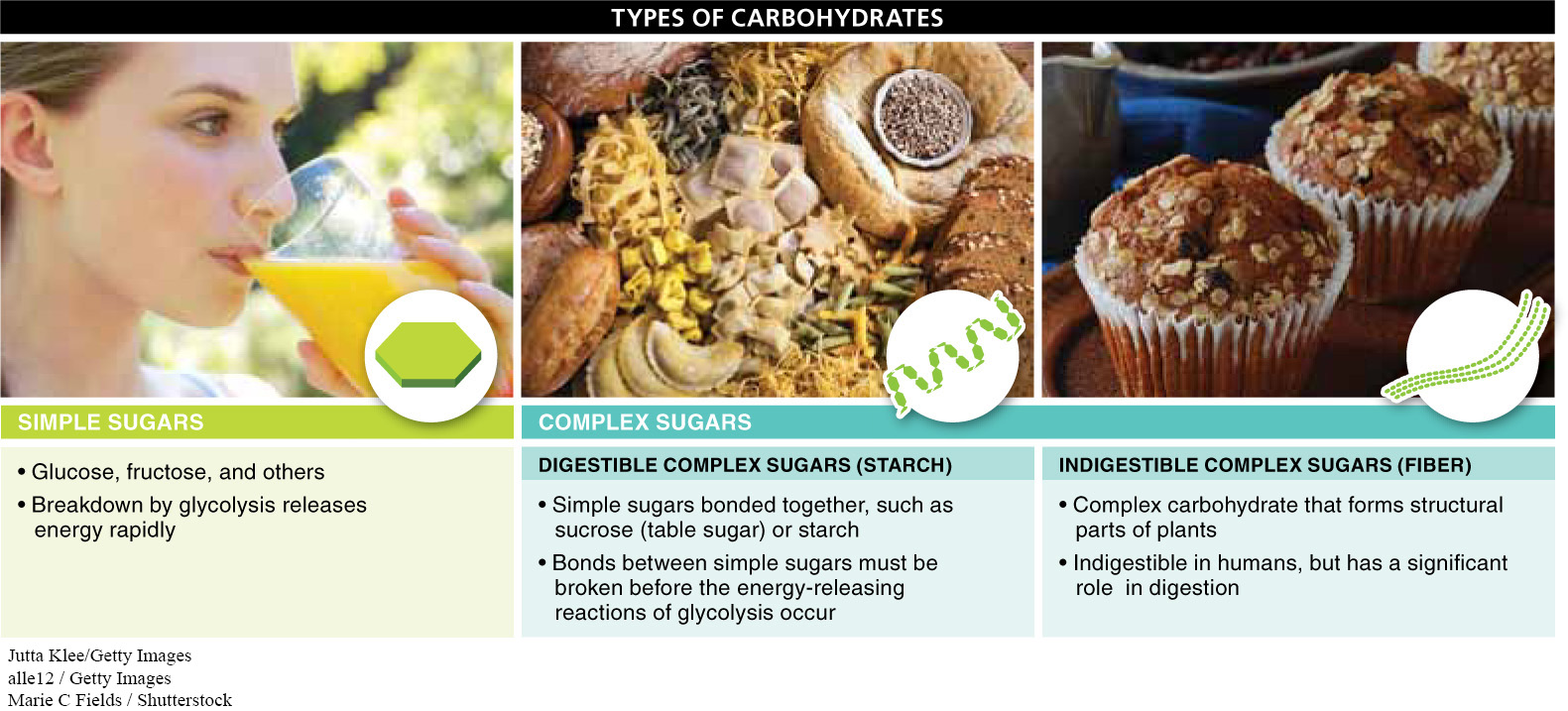
Simple sugars. These include glucose and fructose. They are linear or ring structures with three to seven carbon atoms. Animals can break them down directly through the steps of glycolysis (see Section 4-
Disaccharides and digestible complex sugars. Multiple simple sugars can bond together to form complex but digestible molecules. Disaccharides, such as sucrose (table sugar), are just two simple sugars joined together. Complex sugars, such as starch and glycogen, are large molecules that may consist of hundreds or thousands of glucose molecules connected in dense, branching patterns. For an animal to have access to the energy stored in the bonds of the individual simple sugars, it must first break the bonds that link those sugars together. As these bonds are broken, simple sugars become available for the energy-
883
Fiber. This is a complex carbohydrate, such as cellulose, that forms the structural parts of plants. Fiber differs from starch and other digestible complex sugars by having a different bond connecting the simple sugars together. In humans, this bond cannot be broken by any digestive enzyme, making fiber indigestible. As we’ll see later in this chapter, although fiber isn’t broken down for energy or to supply other molecules used by the body, it still plays an important role in digestion and is necessary in the diet to maintain health.
How and where do humans store carbohydrates? Carbohydrates in our body are stored mostly as glycogen (see Section 2-
Large amounts of water are bound to stored glycogen: 4 pounds of water for every pound of glycogen. Consequently, as glycogen in your liver and muscles is used, the water bound to it is released from the tissue and lost as urine. This is why, as stores of glycogen are depleted in the initial stages of a weight-
Fats: Long-
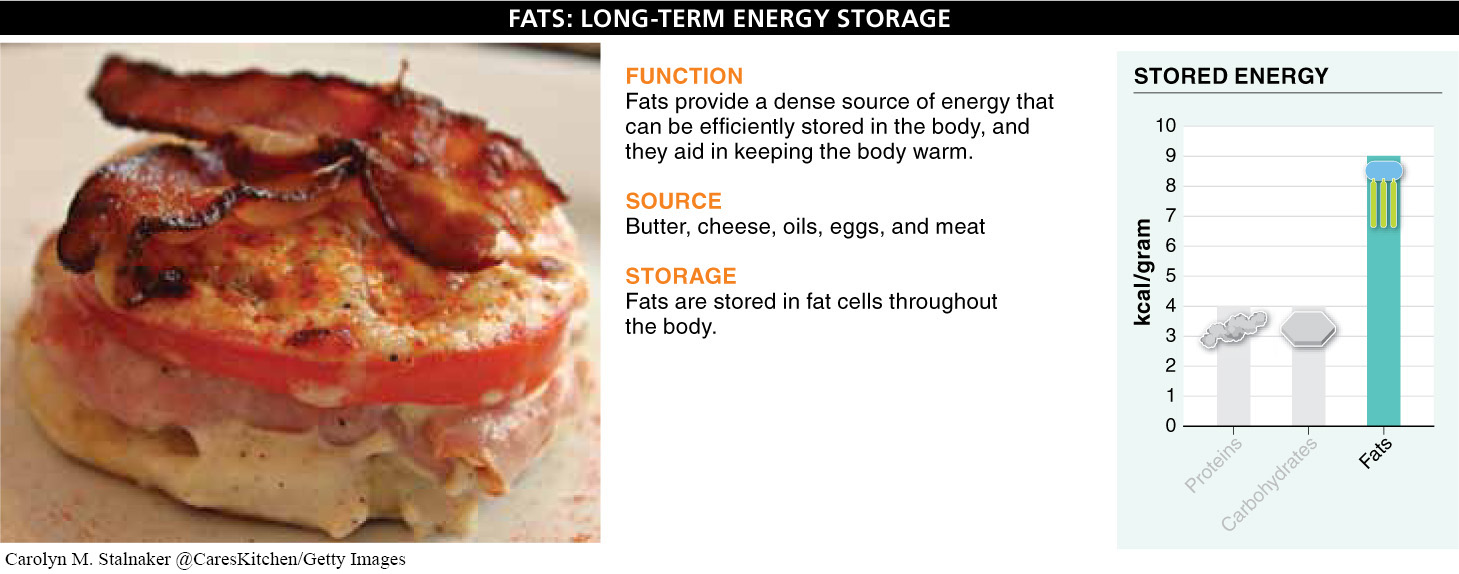
Because fats are poor conductors of heat, fats stored in a layer just beneath the skin can help to keep the body warm. Penguins and walruses, for example, can maintain relatively high body temperatures despite living in very cold habitats, because of their thick layer of insulating fat.
If fats contain more than double the amount of energy found in the same amount of carbohydrates or proteins, why aren’t fats a better fuel to consume before exercising?
Here’s a perplexing fact: although the total amount of energy in a gram of fat is greater than that found in carbohydrates or proteins, fat isn’t the optimum nutrient for most situations. When it comes to exercise, for example, muscle cells need quick access to energy. The problem is that, in muscles, fat burns very slowly for energy. Remember from Chapter 4 that the universal source of chemical energy in the body is ATP. This means that the energy in fat or carbohydrate or protein must be captured as ATP before it is of use to muscle cells. It turns out to be much easier for the body to break down muscle glycogen and blood glucose to make ATP than to break down fat. In fact, the rate of ATP synthesis from carbohydrates is about double the rate from fats.
884
Are all fats the same nutritionally, or do they vary in important ways? In our diet, fats usually come in the form of fatty acids, long chains of carbon atoms with hydrogens attached. With proteins, we saw that there are essential and non-
Another important distinction among dietary fats is between saturated and unsaturated fats (FIGURE 22-12). If each carbon within the chain is bonded to two hydrogen atoms, the molecule carries the maximum number of hydrogen atoms and is said to be saturated. (For a refresher, see Figure 2-31.) Conversely, if some of the carbons are bound to only a single hydrogen, the fatty acid is unsaturated.
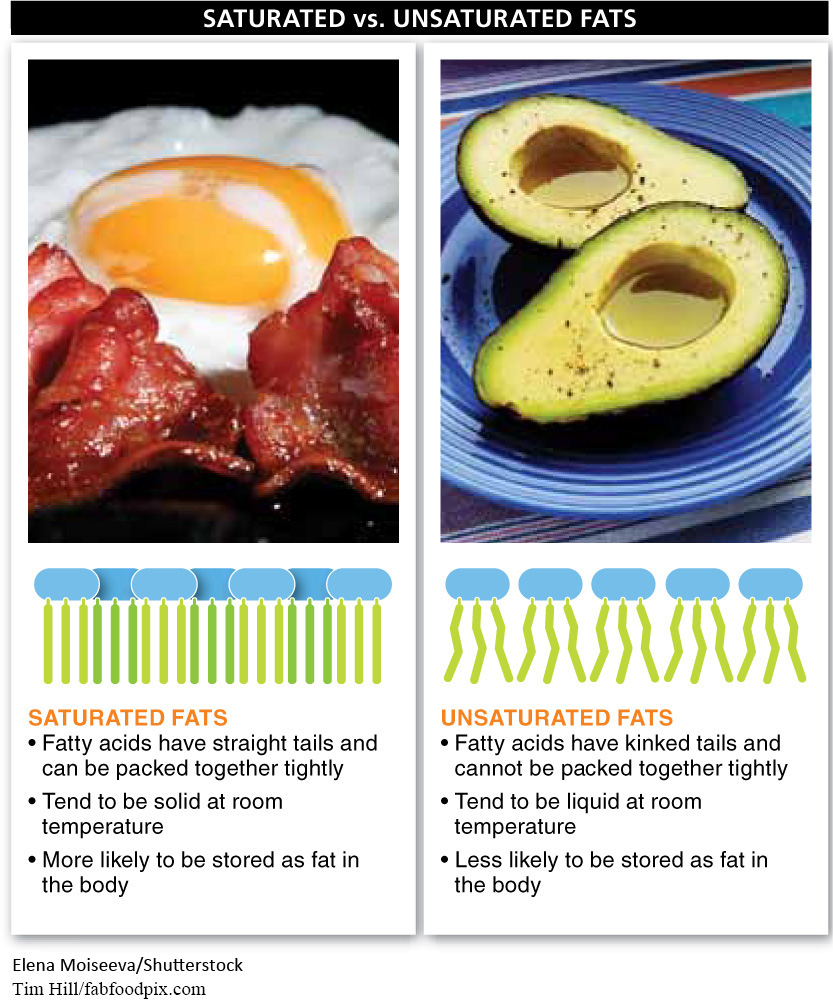
When saturated, fatty acids are very straight and the fat molecules can be packed together tightly. This causes saturated fats to be solid at room temperature, like butter. When unsaturated, the fatty acids have kinks in the hydrocarbon tail and cannot be packed together as tightly. Consequently, unsaturated fats do not solidify so easily and tend to be liquid at room temperature, like vegetable oil. Because unsaturated fatty acids can accept one or more hydrogen atoms, they are a bit less stable and more reactive—
885
Trans fats have been in the news because of their tendency when consumed to raise levels of low-
How and where do we store fats? If a human consumes more calories than he or she burns, most of the excess (regardless of whether these calories were consumed as carbohydrate, fat, or protein) gets converted to fat and stored in fat cells distributed throughout the body. A pound of body fat holds 3,600 kilocalories worth of energy. It is like a savings account for an uncertain future.
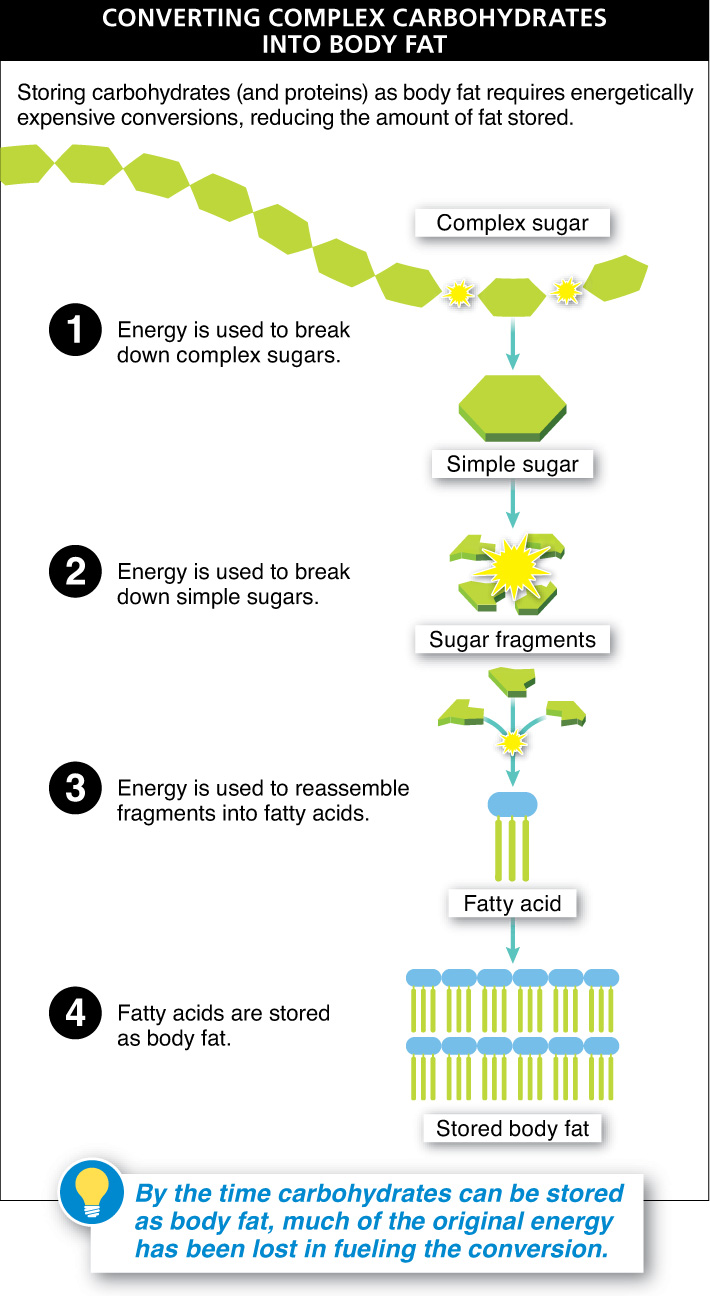
Given that our bodies can convert excess calories into body fat, regardless of whether the calories were initially ingested as carbohydrates or proteins, why is it still an effective weight-
TAKE-HOME MESSAGE 22.6
Carbohydrates are the primary fuel on which animal bodies run. Dietary fats function primarily as a dense source of energy that can be efficiently stored in the body.
What are the three types of carbohydrates described in this chapter?
Three types of carbohydrates include: (1) simple sugars, such as glucose and fructose, (2) digestible complex sugars, such as starch and glycogen, and (3) fiber, which is indigestible but aids in overall digestive health.