When you eat a meal, it is like filling your car’s tank with gasoline; you have a source of energy that can be used to fuel activities like running, thinking, building muscle, and maintaining the machinery of life. That energy initially comes from the sun and is captured and stored by plants. When we eat plants or eat other animals that eat plants, we ingest the energy stored in the plant material. But how exactly is energy stored in plants?
Groups of atoms held together by bonds are called molecules. It takes a certain amount of energy to break a bond between two atoms. The amount of this energy, called the bond energy, depends on the atoms involved. When chemical reactions occur—
Before looking at specific types of bonds that hold molecules together, let’s look at how molecules are illustrated. In this book you will most often see molecular structures represented by the ball-
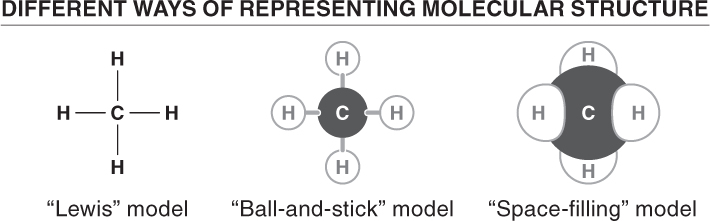
There are three principal types of bonds that hold atoms together. The type of bonding that any atom is likely to take part in depends almost entirely on the number of electrons in its outermost shell.
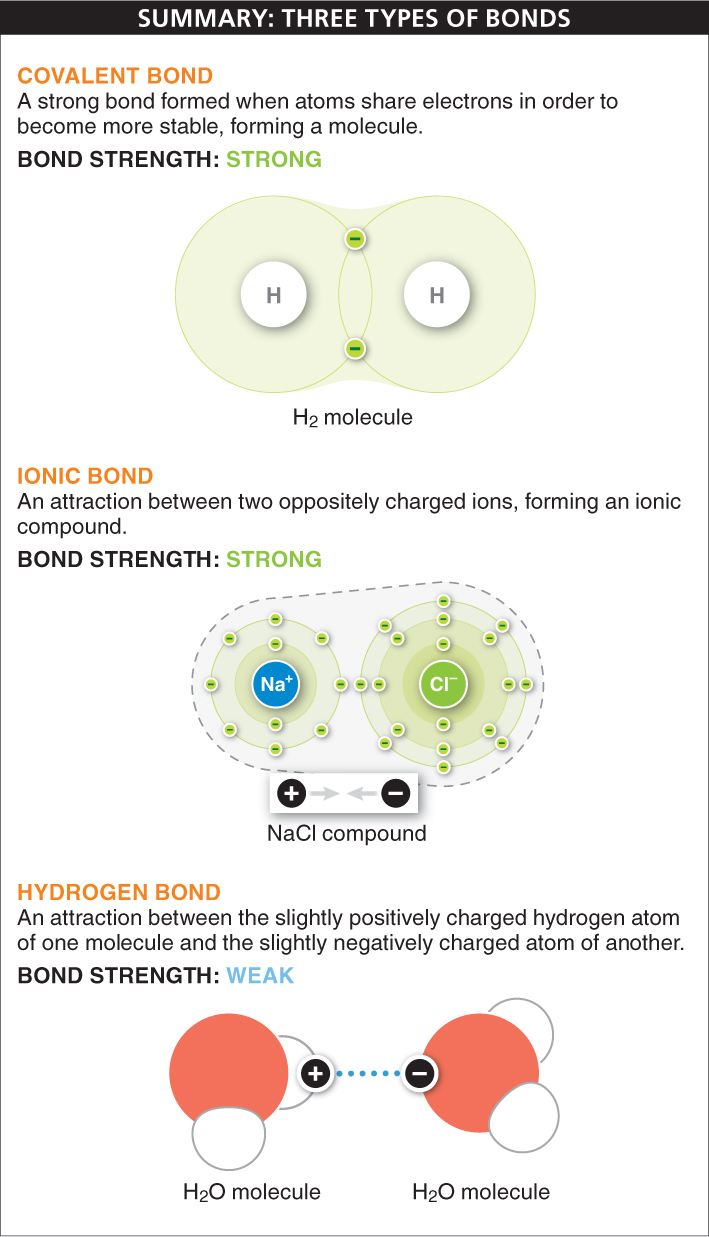
Covalent bonds When two atoms share electrons, a strong covalent bond is formed. The simplest example of a covalent bond is the bonding of two hydrogen atoms to form a hydrogen molecule, H2 (FIGURE 2-9), the simplest of all molecules. A hydrogen atom has an atomic number of 1: it has a single proton in its nucleus and a single electron circling around the nucleus in the first shell. Because the atom is most stable when the first shell has two electrons, two hydrogen atoms can each achieve a complete outermost shell by sharing electrons. The nuclei come close together (but not too close, because they are positively charged and their mutual repulsion would destabilize the molecule), and the two electrons circle around both of the nuclei, almost in a figure 8. The new H2 molecule is very stable because, now that both atoms have two electrons in their outermost shell, they are no longer likely to bond with other atoms. The sharing of two electrons between two atoms forms a single covalent bond.
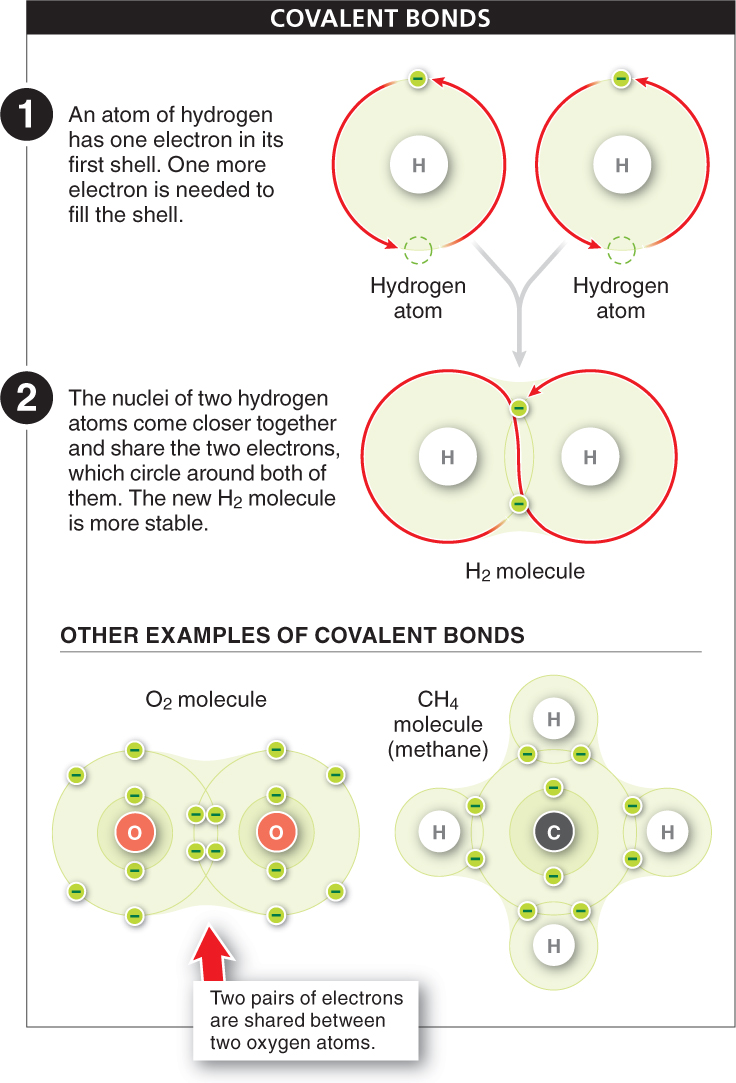
Oxygen, with an atomic number of 8, has two electrons in its innermost shell and six in its outermost shell. Consequently it needs to gain two electrons to fill its outermost shell. Sometimes two oxygen atoms join together to form O2. Each oxygen atom shares a pair of electrons with the other, filling the outermost shell of both. The sharing of two pairs of electrons between two atoms is called a double bond (see Figure 2-
46
Carbon is a particularly “extroverted” atom. It has an atomic number of 6, meaning that two electrons fill its first shell and four remain to occupy the second shell. Because four electron vacancies are left, carbon can, and frequently does, form four covalent bonds, joining up with other atoms in a wide variety of molecules. Methane, the chief component of natural gas, is formed when one atom of carbon covalently bonds with four atoms of hydrogen (see Figure 2-
Ionic bonds Atoms can also bond together without sharing electrons. When one atom transfers one or more of its electrons completely to another, each atom becomes an ion, since each has an unequal number of protons and electrons. The atom gaining electrons becomes negatively charged, while the atom losing electrons becomes positively charged. An ionic bond occurs when the two oppositely charged ions attract each other. Unlike covalent bonds, in ionic bonds each electron circles around a single nucleus. Ions of two or more elements linked by ionic bonds form an ionic compound. Because the ions attracted to each other are of equal and opposite charge, the compound is neutral—
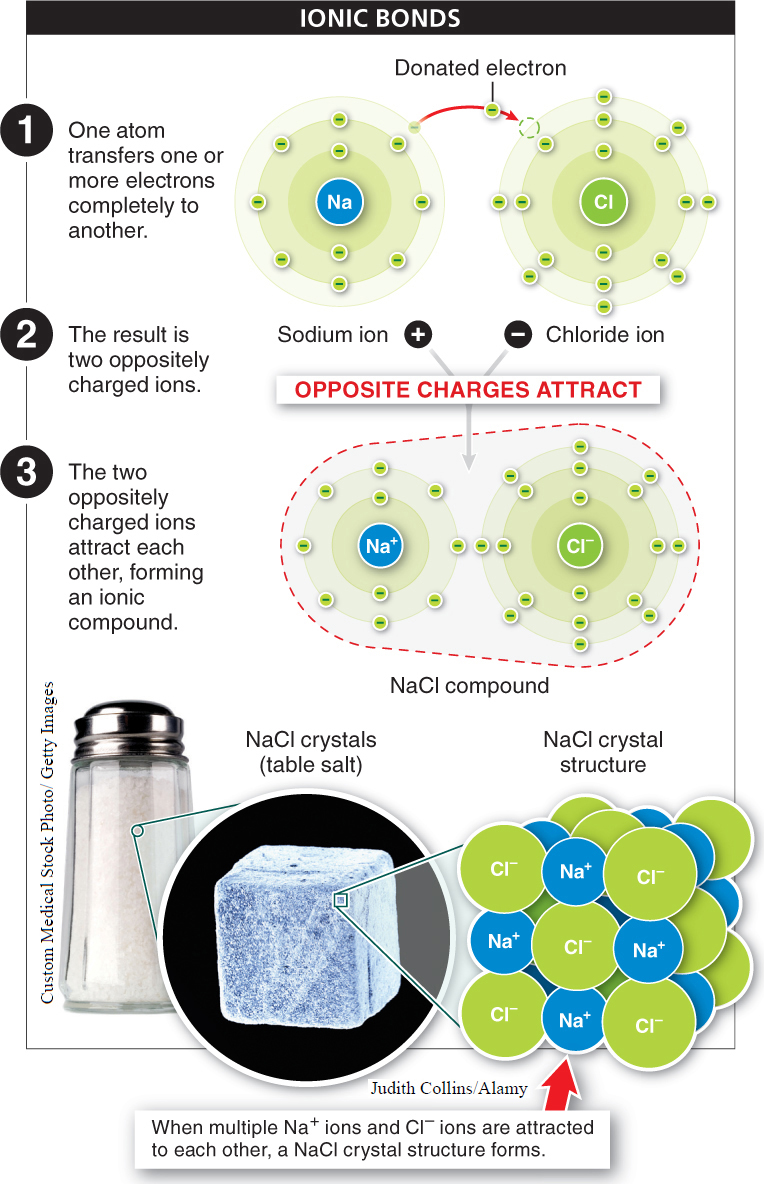
“In the last third of his life, there came over Laszlo Jamf…a hostility, a strangely personal hatred, for the covalent bond…That something so mutable, so soft, as a sharing of electrons by atoms of carbon should be at the core of life, his life, struck Jamf as a cosmic humiliation. Sharing? How much stronger, how everlasting was the ionic bond—
— THOMAS PYNCHON, in Gravity’s Rainbow
[Was Laszlo Jamf mistaken in his understanding of the relative strengths of ionic and covalent bonds?]
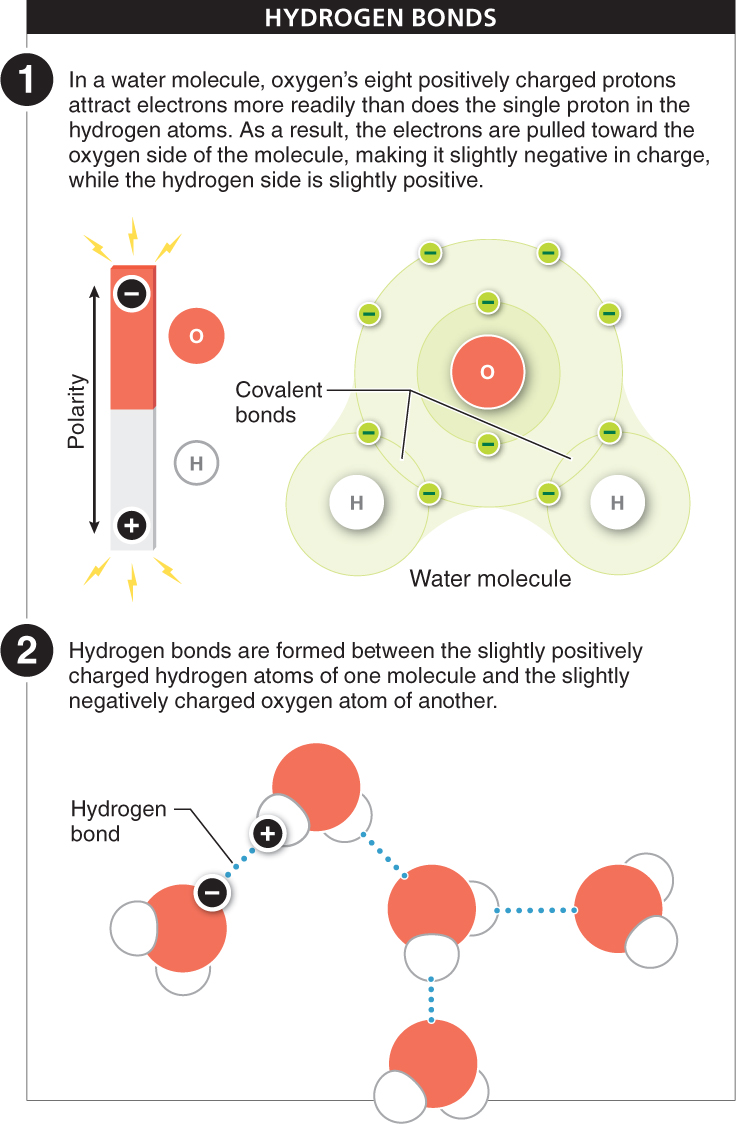
Hydrogen bonds Ionic and covalent bonds link two or more atoms together within an ionic compound or a molecule. Hydrogen bonds, on the other hand, are important in bonding molecules together. A hydrogen bond is formed between a hydrogen atom in one molecule and another atom, often an oxygen or nitrogen atom, in another molecule (or even in another part of the same molecule). This bond is based on the attraction between positive and negative charges.
The atoms taking part in hydrogen bonds are not necessarily ions, so where do the electrical charges come from? The hydrogen atom is already covalently bonded to another atom in the same molecule and shares its electron. That electron circles both the hydrogen nucleus and the nucleus of the other atom, but the electron is not shared equally. It’s as if the nuclei of the atoms are playing tug-
47
In a sense, molecules with slightly charged atoms become like a magnet, with distinct positive and negative sides (see Figure 2-
TAKE-HOME MESSAGE 2.3
Atoms can be bound together in three different ways. Covalent bonds are formed when atoms share electrons. In ionic bonds, one atom transfers its electrons to another and the two oppositely charged ions are attracted to each other, forming an ionic compound. Hydrogen bonds, which are weaker than covalent and ionic bonds, are formed from the attraction between a hydrogen atom and another atom with a slight negative charge.
Describe and contrast covalent and ionic bonds.
Covalent bonds are formed when two atoms share a pair of electrons. Ionic bonds are formed when one or more electrons are transferred from one atom to another, forming ions of opposite charges that are attracted to each other.
48