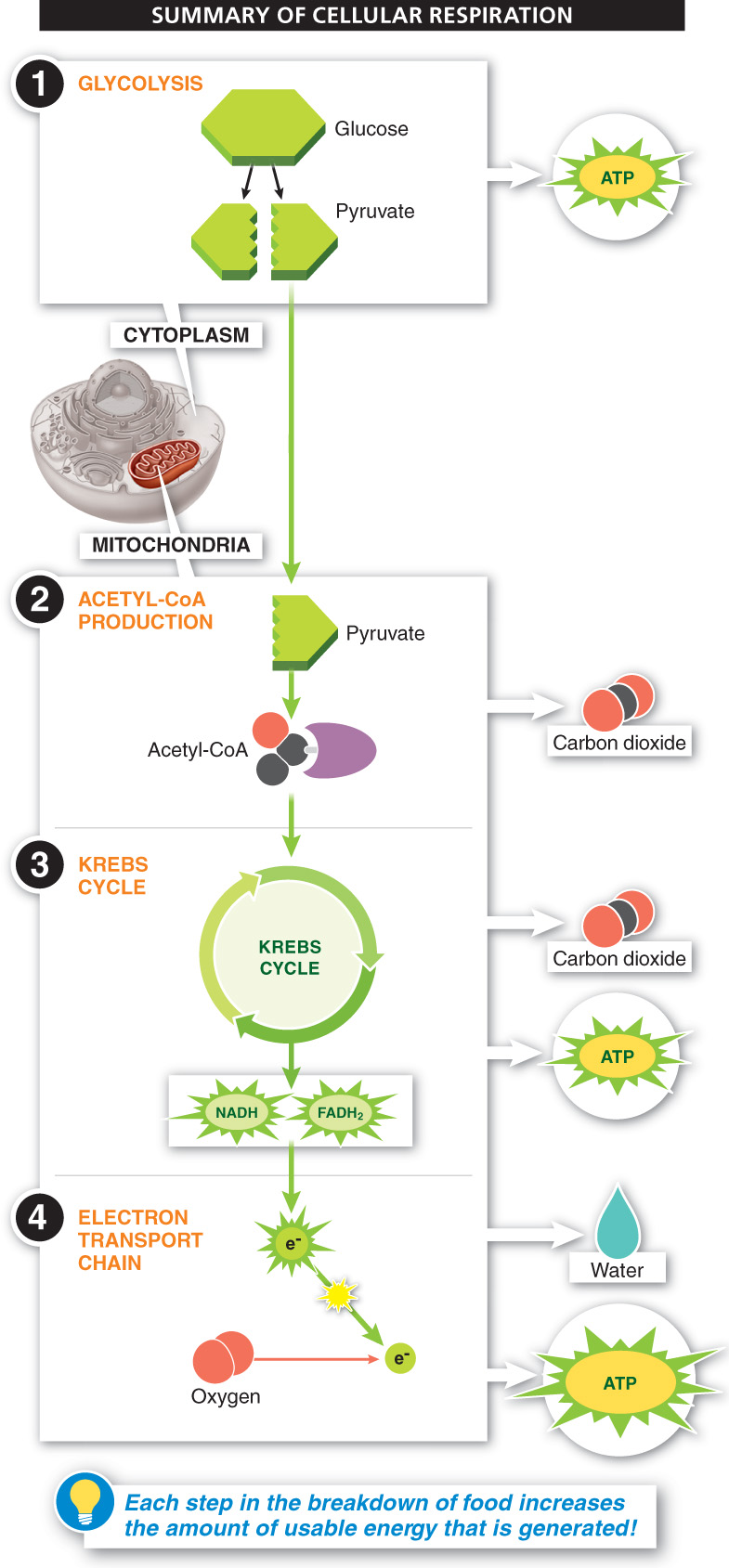
How do we finally get a big payoff of usable energy from our glucose molecule? Glycolysis and the Krebs cycle produce a few molecules of ATP for each molecule of glucose broken down, but it is the energy held in the high-
In a manner similar to that seen in the chloroplast during photosynthesis, mitochondria convert kinetic energy (from electrons) into potential energy (a concentration gradient of protons). Two structural features of mitochondria are essential to their impressive ability to harness energy from molecules.
Feature 1. Mitochondria have a “bag-
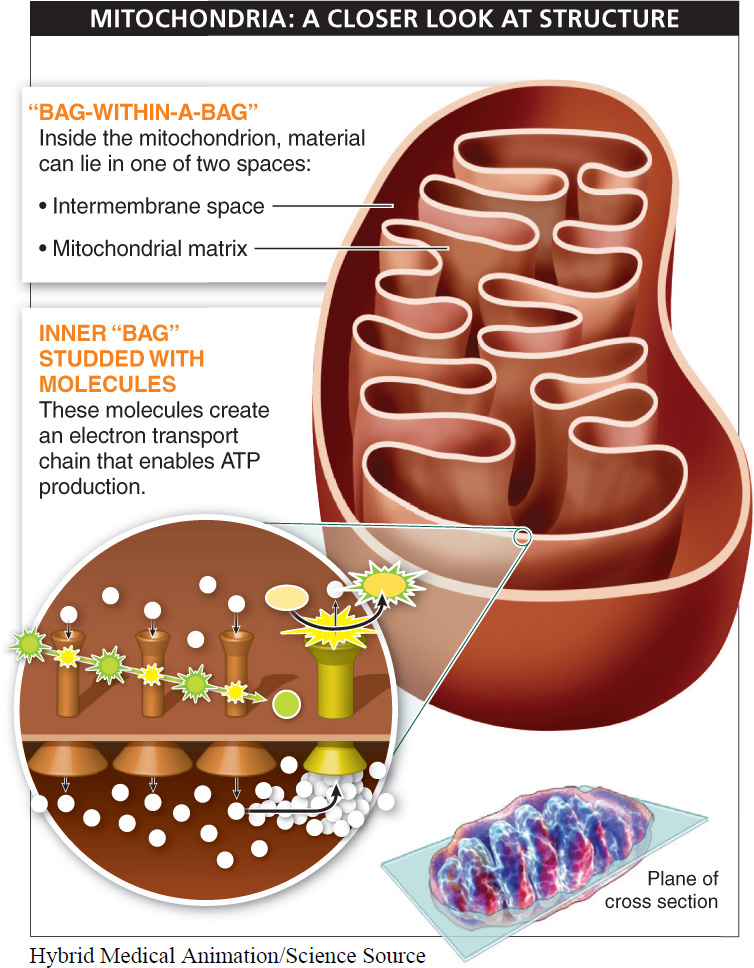
Material inside the mitochondrion can lie in one of two spaces: (1) in the intermembrane space, which is outside the inner bag, or (2) in the mitochondrial matrix, which is inside the inner bag. With two distinct regions separated by a membrane, the mitochondrion can create higher concentrations of molecules in one area or the other, creating a concentration gradient. And because a concentration gradient is a form of potential energy—
163
Feature 2. The inner bag of the mitochondrion is studded with molecules, mostly electron carriers, which are sequentially arranged as a “chain.” This arrangement makes it possible for the molecules to hand off electrons in an orderly sequence.
Now let’s explore how these features of mitochondria make it possible to harness energy from high-
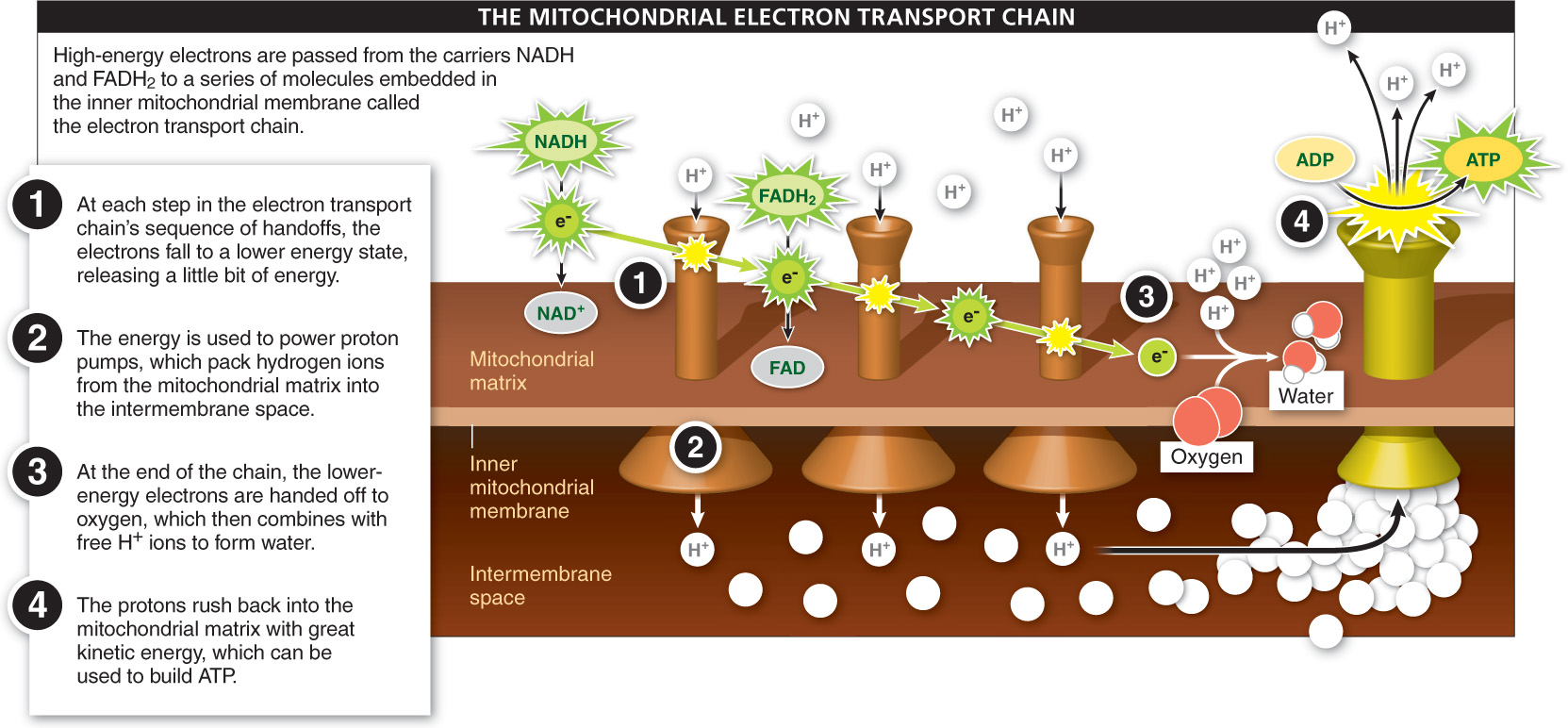
Over-
Step 1 of the electron transport chain begins with NADH and FADH2 in the mitochondrial matrix (inside the inner bag) moving to the membrane. There, the high-
The membrane-
At the end of the chain (step 2 in Figure 4-
As shown in step 3 of Figure 4-
If this description seems familiar, it is. In chloroplasts, during photosynthesis, great numbers of protons are pumped from the stroma outside the thylakoid sacs to the inside of the thylakoids. We likened this potential energy to the potential energy of water in an elevated tower, which can be released with great force. Similarly, in step 4 of the mitochondrial electron transport chain, the protons pumped into the intermembrane space rush back into the mitochondrial matrix through channels in the inner mitochondrial membrane. And as the protons pass through, the force of their flow fuels the attachment of free-
164
In the end, the number of ATP molecules generated from the complete dismantling of one molecule of glucose is about 36, most of which are produced with the energy harnessed from high-
Cyanide blocks the passage of electrons to oxygen in the electron transport chain. Why does this make it a toxic poison?
Given the central role of the electron transport chain in the generation of usable energy from the breakdown of food molecules, any interference in its functioning has dire consequences. And in fact, murder by cyanide poisoning, an old tradition in detective stories, is just such an interference. When cyanide gets into the mitochondria, it binds to a molecule in the electron transport chain, preventing it from accepting electrons. This halts the transfer of electrons and the pumping of protons across the mitochondrial membrane. As a consequence, the production of ATP that would occur when protons rushed back across the membrane down their concentration gradient ceases. Halting the production of ATP removes a cell’s energy source, starving it very quickly. For this reason, cyanide poisoning can cause death within minutes.
TAKE-HOME MESSAGE 4.15
The largest energy payoff of cellular respiration comes as electrons from the NADH and FADH2 produced during glycolysis and the Krebs cycle move along the electron transport chain. The electrons are passed from one carrier to another and energy is released, pumping protons into the mitochondrial intermembrane space. As the protons rush back into the mitochondrial matrix, the force of their flow fuels the production of large amounts of ATP.
What is the ultimate fate of the electrons passed through the electron transport chain?
Energy is released from electrons while they travel through the electron transport chain, which ultimately leads to the formation of most of the ATP needed by the cell. These low-energy electrons are ultimately combined with oxygen and hydrogen ions to form water.
165