14.3 Transfer RNAs, Which Attach to Amino Acids, Are Modified after Transcription in Bacterial and Eukaryotic Cells
In 1956, Francis Crick proposed the idea of a molecule that transports amino acids to the ribosome and interacts with codons in mRNA, placing amino acids in their proper order in protein synthesis. By 1963, the existence of such an adapter molecule, called transfer RNA, had been confirmed. Transfer RNA (tRNA) serves as a link between the genetic code in mRNA and the amino acids that make up a protein. Each tRNA attaches to a particular amino acid and carries it to the ribosome, where the tRNA adds its amino acid to the growing polypeptide chain at the position specified by the genetic instructions in the mRNA. We’ll take a closer look at the mechanism of this process in Chapter 15.
398
Each tRNA is capable of attaching to only one type of amino acid. The complex of tRNA plus its amino acid can be written in abbreviated form by adding a three-letter superscript representing the amino acid to the term tRNA. For example, a tRNA that attaches to the amino acid alanine is written as tRNAAla. Because 20 different amino acids are found in proteins, there must be a minimum of 20 different types of tRNA. In fact, most organisms possess at least 30 to 40 different types of tRNA, each encoded by a different gene (or, in some cases, multiple copies of a gene) in DNA.
The Structure of Transfer RNA
A unique feature of tRNA is the occurrence of rare modified bases. All RNAs have the four standard bases (adenine, cytosine, guanine, and uracil) specified by DNA, but tRNAs have additional bases, including ribothymine, pseudouridine (which is also occasionally present in snRNAs and rRNA), and dozens of others. The structures of two of these modified bases are shown in Figure 14.18.
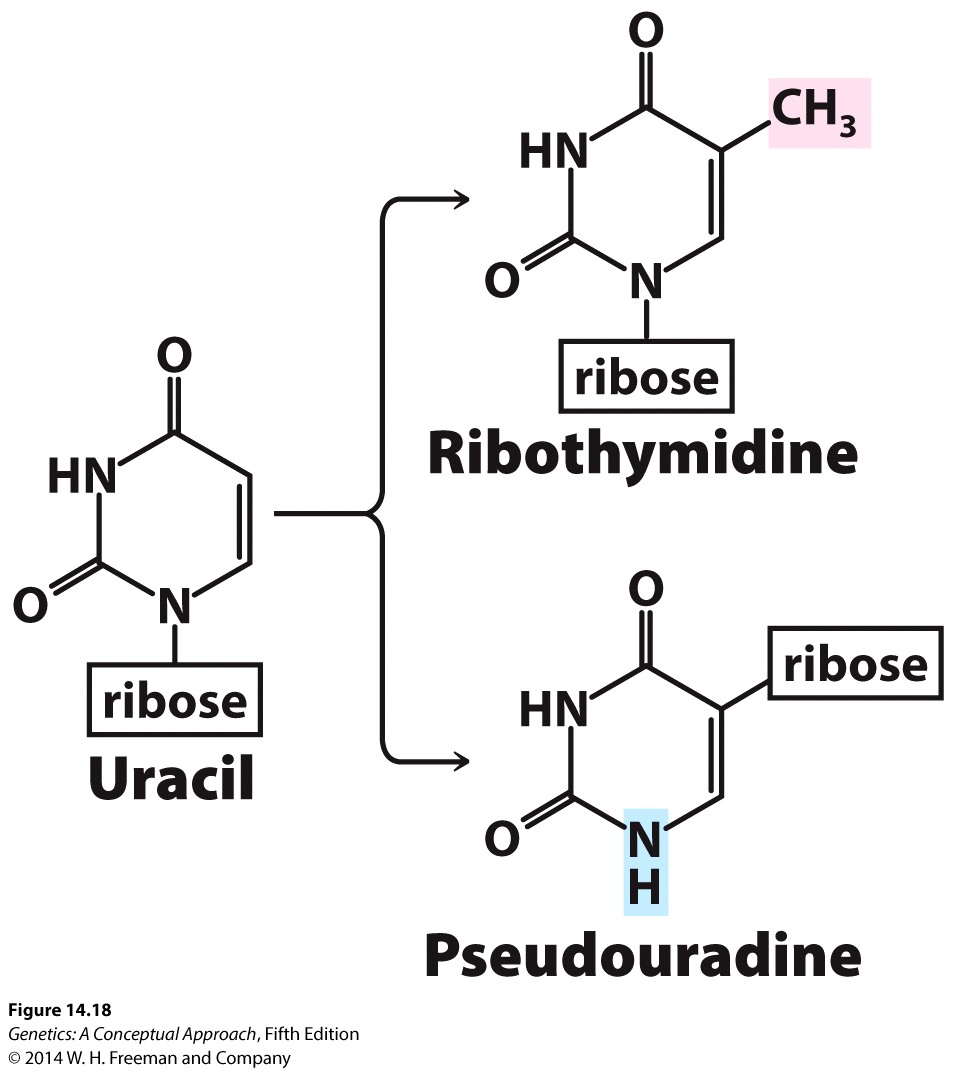
If there are only four bases in DNA and all RNA molecules are transcribed from DNA, how do tRNAs acquire these additional bases? Modified bases arise from chemical changes made to the four standard bases after transcription. These changes are carried out by special tRNA-modifying enzymes. For example, the addition of a methyl group to uracil creates the modified base ribothymidine.
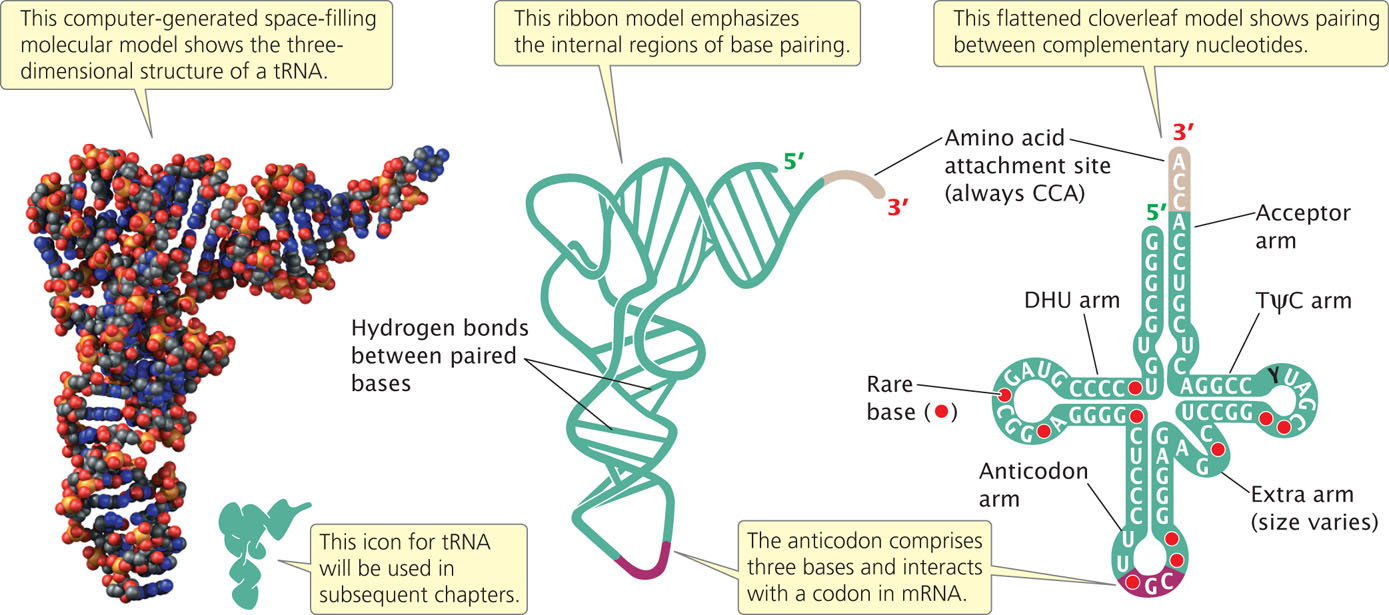
The structures of all tRNAs are similar, a feature critical to tRNA function. Most tRNAs contain between 74 and 95 nucleotides, some of which are complementary to each other and form intramolecular hydrogen bonds. As a result, each tRNA has a cloverleaf structure (Figure 14.19). The cloverleaf has four major arms. If we start at the top and proceed clockwise around the tRNA shown at the right in Figure 14.19, the four major arms are the acceptor arm, the TΨC arm, the anticodon arm, and the DHU arm. Three of the arms (the TΨC, anticodon, and DHU arms) consist of a stem and a loop. The stem is formed by the pairing of complementary nucleotides, and the loop lies at the terminus of the stem, where there is no nucleotide pairing.
399
Instead of having a loop, the acceptor arm includes the 5′ and 3′ ends of the tRNA molecule. All tRNAs have the same sequence (CCA) at the 3′ end, where the amino acid attaches to the tRNA; so, clearly, this sequence is not responsible for specifying which amino acid will attach to the tRNA.
The TΨC arm is named for the bases of three nucleotides in the loop of this arm: thymine (T), pseudouridine (Ψ), and cytosine (C). The anticodon arm lies at the bottom of the tRNA. Three nucleotides at the end of this arm make up the anticodon, which pairs with the corresponding codon on mRNA to ensure that the amino acids link in the correct order. The DHU arm is so named because it often contains the modified base dihydrouridine.
Although each tRNA molecule folds into a cloverleaf owing to the complementary pairing of bases, the cloverleaf is not the three-dimensional (tertiary) structure of tRNAs found in the cell. The results of X-ray crystallographic studies have shown that the cloverleaf folds on itself to form an L-shaped structure, as illustrated by the space-filling and ribbon models in Figure 14.19. Notice that the acceptor stem is at one end of the tertiary structure and the anticodon is at the other end.
Transfer RNA Gene Structure and Processing
The genes that produce tRNAs may be in clusters or scattered about the genome. In E. coli, the genes for some tRNAs are present in a single copy, whereas the genes for other tRNAs are present in several copies; eukaryotic cells usually have many copies of each tRNA gene. All tRNA molecules in both bacterial and eukaryotic cells undergo processing after transcription.
In E. coli, several tRNAs are usually transcribed together as one large precursor tRNA, which is then cut up into pieces, each containing a single tRNA. Additional nucleotides may then be removed one at a time from the 5′ and 3′ ends of the tRNA in a process known as trimming. Base-modifying enzymes may then change some of the standard bases into modified bases (Figure 14.20). In some prokaryotes, the CCA sequences found at the 3′ ends of tRNAs are encoded in the tRNA gene and are transcribed into the tRNA; in other prokaryotes and in eukaryotes, these sequences are added by a special enzyme that adds the nucleotides without the use of any template.
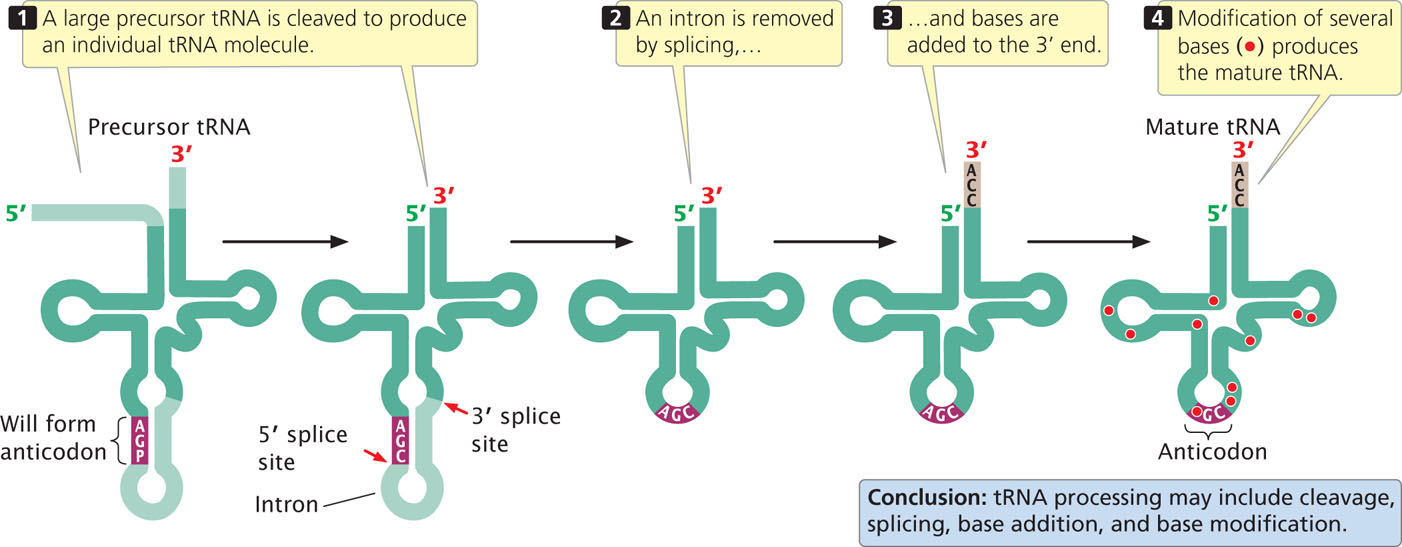
There is no generic processing pathway for all tRNAs: different tRNAs are processed in different ways. Eukaryotic tRNAs are processed in a manner similar to that for bacterial tRNAs: most are transcribed as larger precursors that are then cleaved, trimmed, and modified to produce mature tRNAs.
Some eukaryotic and archaeal tRNA genes possess introns of variable length that must be removed in processing. For example, about 40 of the 400 tRNA genes in yeast contain a single intron that is always found adjacent to the 3′ side of the anticodon. The tRNA introns are shorter than those found in pre-mRNA and do not have the consensus sequences found at the intron-exon junctions of pre-mRNAs. The splicing process for tRNA genes is quite different from the spliceosome-mediated reactions that remove introns from protein-encoding genes.
CONCEPTS
All tRNAs are similar in size and have a common secondary structure known as the cloverleaf. Transfer RNAs contain modified bases and are extensively processed after transcription in both bacterial and eukaryotic cells.
400
 CONCEPT CHECK 7
CONCEPT CHECK 7
How are rare bases incorporated into tRNAs?
- Encoded by guide RNAs
- By chemical changes to one of the standard bases
- Encoded by rare bases in DNA
- Encoded by sequences in introns