Application Questions and Problems
Section 16.1
Question 16.10
Examine Figure 16.2b. Why do you think the motif of the DNA binding protein shown is called a zinc finger protein?
Section 16.2
Question 16.11
For each of the following types of transcriptional control, indicate whether the protein produced by the regulator gene will be synthesized initially as an active repressor, inactive repressor, active activator, or inactive activator.
- a. Negative control in a repressible operon
- b. Positive control in a repressible operon
- c. Negative control in an inducible operon
- d. Positive control in an inducible operon
Question 16.12
A mutation at the operator site prevents the regulator protein from binding. What effect will this mutation have in the following types of operons?
- a. Regulator protein is a repressor in a repressible operon.
- b. Regulator protein is a repressor in an inducible operon.
Question 16.13
The blob operon produces enzymes that convert compound A into compound B. The operon is controlled by a regulatory gene S. Normally, the enzymes are synthesized only in the absence of compound B. If gene S is mutated, the enzymes are synthesized in the presence and in the absence of compound B. Does gene S produce a repressor or an activator? Is this operon inducible or repressible?
Question 16.14
A mutation prevents the catabolite activator protein (CAP) from binding to the promoter in the lac operon. What will the effect of this mutation be on the transcription of the operon?
Question 16.15
Transformation is a process in which bacteria take up new DNA released by dead cells and integrate it into their own genomes. In Streptococcus pneumonia (which causes many cases of pneumonia, inner-ear infections, and meningitis) the ability to carry out transformation requires from 105 to 124 genes, collectively termed the com regulon. The com regulon is activated in response to a protein called competence-stimulating peptide (CSP), which is produced by bacteria and is exported into the surrounding medium. When enough CSP accumulates, it attaches to a receptor on the bacterial cell membrane, which then activates a regulator protein that stimulates the transcription of genes within the com regulon and sets in motion a series of reactions that ultimately results in transformation. The com regulon in Streptococcus pneumoniae appears to be controlled through which type of gene regulation? Explain your answer.
- a. Negative inducible
- b. Negative repressible
- c. Positive inducible
- d. Positive repressible
Question 16.16
Under which of the following conditions would a lac operon produce the greatest amount of β-glactosidase? The least? Explain your reasoning.
| Lactose present | Glucose present | |
|---|---|---|
| Condition 1 | Yes | No |
| Condition 2 | No | Yes |
| Condition 3 | Yes | Yes |
| Condition 4 | No | No |
Question 16.17
A mutant strain of E. coli produces β-galactosidase in both the presence and the absence of lactose. Where in the operon might the mutation in this strain occur?
Question 16.18
Examine Figure 16.8. What would be the effect of a drug that altered the structure of allolactose so that it was unable to bind to the regulator protein?
Question 16.19
For E. coli strains with the lac genotypes use a plus sign (+) to indicate the synthesis of β-galactosidase and permease and a minus sign (−) to indicate no synthesis of the proteins.
| Lactose absent | Lactose present | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotype of strain | β-Galactosidase | Permease | β-Galactosidase | Permease | ||||
| lacI+ | lacP+ | lacO+ | lacZ+ | lacY+ | ||||
| lacI~ | lacP+ | lacO+ | lacZ+ | lacY+ | ||||
| lacI+ | lacP+ | lacOc | lacZ+ | lacY+ | ||||
| lacI− | lacP+ | lacO+ | lacZ+ | lacY− | ||||
| lacI− | lacP− | lacO+ | lacZ+ | lacY+ | ||||
| lacI+ | lacP+ | lacO+ | lacZ− | lacY+/ | ||||
| lacI− | lacP+ | lacO+ | lacZ+ | lacY− | ||||
| lacI− | lacP+ | lacOc | lacZ+ | lacY+/ | ||||
| lacI+ | lacP+ | lacO+ | lacZ− | lacY− | ||||
| lacI− | lacP+ | lacO+ | lacZ+ | lacY−/ | ||||
| lacI+ | lacP− | lacO+ | lacZ− | lacY+ | ||||
| lacI+ | lacP− | lacOc | lacZ− | lacY+/ | ||||
| lacI− | lacP+ | lacO+ | lacZ+ | lacY− | ||||
| lacI+ | lacP+ | lacO+ | lacZ+ | lacY+/ | ||||
| lacI+ | lacP+ | lacO+ | lacZ+ | lacY+ | ||||
| lacIs | lacP+ | lacO+ | lacZ+ | lacY−/ | ||||
| lacI+ | lacP+ | lacO+ | lacZ− | lacY+ | ||||
| lacIs | lacP− | lacO+ | lacZ− | lacY+/ | ||||
| lacI+ | lacP+ | lacO+ | lacZ+ | lacY+ | ||||
Question 16.20
Give all possible genotypes of a lac operon that produces β-galactosidase and permease under the following conditions. Do not give partial diploid genotypes.
| Lactose absent | Lactose present | |||
|---|---|---|---|---|
| β-Galactosidase | Permease | β-Galactosidase | Permease | |
| a. | − | − | + | + |
| b. | − | − | − | + |
| c. | − | − | + | − |
| d. | + | + | + | + |
| e. | − | − | − | − |
| f. | + | − | + | − |
| g. | − | + | − | + |
Question 16.21
Explain why mutations in the lacI gene are trans in their effects, but mutations in the lacO gene are cis in their effects.
Question 16.22
Which strand of DNA (upper or lower) in Figure 16.9 is the template strand? Explain your reasoning.
Question 16.23
The mmm operon, which has sequences A, B, C, and D (which may be structural genes or regulatory sequences), encodes enzymes 1 and 2. Mutations in sequences A, B, C, and D have the following effects, where a plus sign (+) indicates that the enzyme is synthesized and a minus sign (−) indicates that the enzyme is not synthesized.
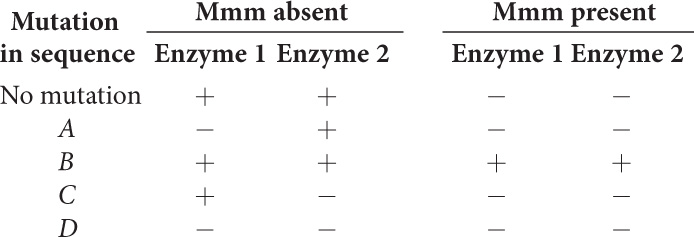
|
- a. Is the mmm operon inducible or repressible?
- b. Indicate which sequence (A, B, C, or D) is part of the following components of the operon:
Regulator gene ____________ Promoter ____________ Structural gene for enzyme 1 ____________ Structural gene for enzyme 2 ____________
Question 16.24
 Ellis Engelsberg and his coworkers examined the regulation of genes taking part in the metabolism of arabinose, a sugar (E. Engelsberg et al. 1965. Journal of Bacteriology 90:946–957). Four structural genes encode enzymes that help metabolize arabinose (genes A, B, D, and E). An additional gene C is linked to genes A, B, and D. These genes are in the order D-A-B-C. Gene E is distant from the other genes. Engelsberg and his colleagues isolated mutations at the C gene that affected the expression of structural genes A, B, D, and E. In one set of experiments, they created various genotypes at the A and C loci and determined whether arabinose isomerase (the enzyme encoded by gene A) was produced in the presence or absence of arabinose (the substrate of arabinose isomerase). Results from this experiment are shown in the following table, where a plus sign (+) indicates that the arabinose isomerase was synthesized and a minus sign (−) indicates that the enzyme was not synthesized.
Ellis Engelsberg and his coworkers examined the regulation of genes taking part in the metabolism of arabinose, a sugar (E. Engelsberg et al. 1965. Journal of Bacteriology 90:946–957). Four structural genes encode enzymes that help metabolize arabinose (genes A, B, D, and E). An additional gene C is linked to genes A, B, and D. These genes are in the order D-A-B-C. Gene E is distant from the other genes. Engelsberg and his colleagues isolated mutations at the C gene that affected the expression of structural genes A, B, D, and E. In one set of experiments, they created various genotypes at the A and C loci and determined whether arabinose isomerase (the enzyme encoded by gene A) was produced in the presence or absence of arabinose (the substrate of arabinose isomerase). Results from this experiment are shown in the following table, where a plus sign (+) indicates that the arabinose isomerase was synthesized and a minus sign (−) indicates that the enzyme was not synthesized.
| Genotype | Arabinose absent | Arabinose present | |
|---|---|---|---|
| 1. | C+ A+ | − | + |
| 2. | C− A+ | − | − |
| 3. | C− A+/C+ A− | − | + |
| 4. | Cc A−/C− A+ | + | + |
- a. On the basis of the results of these experiments, is the C gene an operator or a regulator gene? Explain your reasoning.
- b. Do these experiments suggest that the arabinose operon is negatively or positively controlled? Explain your reasoning.
- c. What type of mutation is Cc?
Question 16.25
 In E. coli, three structural genes (A, D, and E) encode enzymes A, D, and E respectively. Gene O is an operator. The genes are in the order O-A-D-E on the chromosome. These enzymes catalyze the biosynthesis of valine. Mutations were isolated at the A, D, E, and O genes to study the production of enzymes A, D, and E when cellular levels of valine were low (T. Ramakrishnan and E. A. Adelberg. 1965. Journal of Bacteriology 89:654-660). Levels of the enzymes produced by partial-diploid E. coli with various combinations of mutations are shown in the following table.
In E. coli, three structural genes (A, D, and E) encode enzymes A, D, and E respectively. Gene O is an operator. The genes are in the order O-A-D-E on the chromosome. These enzymes catalyze the biosynthesis of valine. Mutations were isolated at the A, D, E, and O genes to study the production of enzymes A, D, and E when cellular levels of valine were low (T. Ramakrishnan and E. A. Adelberg. 1965. Journal of Bacteriology 89:654-660). Levels of the enzymes produced by partial-diploid E. coli with various combinations of mutations are shown in the following table.
| Amount of enzyme produced | ||||
|---|---|---|---|---|
| Genotype | E | D | A | |
| 1. | E+ D+ A+ O+/ | 2.40 | 2.00 | 3.50 |
| E+ D+ A+ O+ | ||||
| 2. | E+ D+ A+ O− / | 35.80 | 38.60 | 46.80 |
| E+ D+ A+ O+ | ||||
| 3. | E+ D− A+ O− / | 1.80 | 1.00 | 47.00 |
| E+ D+ A− O+ | ||||
| 4. | E+ D+ A− O− / | 35.30 | 38.00 | 1.70 |
| E+ D− A+ O+ | ||||
| 5. | E− D+ A+ O− / | 2.38 | 38.00 | 46.70 |
| E+ D− A+ O+ | ||||
- a. Is the regulator protein that binds to the operator of this operon a repressor (negative control) or an activator (positive control)? Explain your reasoning.
- b. Are genes A, D, and E all under the control of operator O? Explain your reasoning.
- c. Propose an explanation for the low level of enzyme E produced in genotype 3.
Section 16.3
Question 16.27
Listed in parts a through g are some mutations that were found in the 5′ UTR region of the trp operon of E. coli. What will the most likely effect of each of these mutations be on the transcription of the trp structural genes?
- a. A mutation that prevents the binding of the ribosome to the 5′ end of the mRNA 5′ UTR
- b. A mutation that changes the tryptophan codons in region 1 of the mRNA 5′ UTR into codons for alanine
- c. A mutation that creates a stop codon early in region 1 of the mRNA 5′ UTR
- d. Deletions in region 2 of the mRNA 5′ UTR
- e. Deletions in region 3 of the mRNA 5′ UTR
- f. Deletions in region 4 of the mRNA 5′ UTR
- g. Deletion of the string of adenine nucleotides that follows region 4 in the 5′ UTR
Question 16.28
Some mutations in the trp 5′ UTR region increase termination by the attenuator. Where might these mutations occur and how might they affect the attenuator?
Question 16.29
Some of the mutations mentioned in Problem 28 have an interesting property. They prevent the formation of the antiterminator that normally takes place when the tryptophan level is low. In one of the mutations, the AUG start codon for the 5′ UTR peptide has been deleted. How might this mutation prevent antitermination from taking place?
Section 16.4
Question 16.30
Several examples of antisense RNA regulating translation in bacterial cells have been discovered. Molecular geneticists have also used antisense RNA to artificially control transcription in both bacterial and eukaryotic genes. If you wanted to inhibit the transcription of a bacterial gene with antisense RNA, what sequences might the antisense RNA contain?