17.4 Some Genes Are Regulated by RNA Processing and Degradation
In bacteria, transcription and translation take place simultaneously. In eukaryotes, transcription takes place in the nucleus and the pre-mRNAs are then processed before moving to the cytoplasm for translation, allowing opportunities for gene control after transcription. Consequently, posttranscriptional gene regulation assumes an important role in eukaryotic cells. A common level of gene regulation in eukaryotes is RNA processing and degradation.
Gene Regulation Through RNA Splicing
Alternative splicing allows a pre-mRNA to be spliced in multiple ways, generating different proteins in different tissues or at different times in development (see Chapter 14). Many eukaryotic genes undergo alternative splicing, and the regulation of splicing is an important means of controlling gene expression in eukaryotic cells.
Alternative Splicing in the T-Antigen Gene
The T-antigen gene of the mammalian virus SV40 is a well-studied example of alternative splicing. This gene is capable of encoding two different proteins, the large T and small t antigens. Which of the two proteins is produced depends on which of two alternative 5′ splice sites is used in RNA splicing (Figure 17.10,). The use of one 5′ splice site produces mRNA that encodes the large T antigen, whereas the use of the other 5′ splice site (which is farther downstream) produces an mRNA encoding the small t antigen.
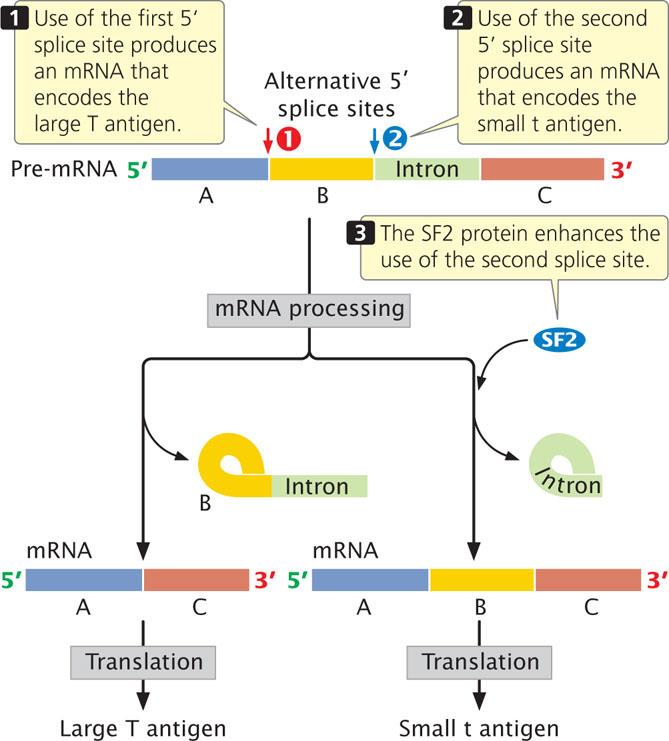
A protein called splicing factor 2 (SF2) enhances the production of mRNA encoding the small t antigen (see Figure 17.10). Splicing factor 2 has two binding domains: one domain is an RNA-binding region and the other has alternating serine and arginine amino acids. These two domains are typical of SR (serine- and arginine-rich) proteins, which often play a role in regulating splicing. Splicing factor 2 stimulates the binding of small nuclear ribonucleoproteins (snRNPs) to the 5′ splice site, one of the earliest steps in RNA splicing (see Chapter 14). The precise mechanism by which SR proteins influence the choice of splice sites is poorly understood. One model suggests that SR proteins bind to specific splice sites on mRNA and stimulate the attachment of snRNPs, which then commit the site to splicing.
Alternative Splicing in Drosophila Sexual Development
Another example of alternative mRNA splicing that regulates gene expression is the determination of whether a fruit fly develops as male or female. Sex differentiation in Drosophila arises from a cascade of gene regulation. When two X-chromosomes are present, a female-specific promoter is activated early in development and stimulates the transcription of the sex-lethal (Sxl) gene (Figure 17.11). The protein encoded by Sxl regulates the splicing of the pre-mRNA transcribed from another gene called transformer (tra). The splicing of tra pre-mRNA results in the production of the Tra protein (see Figure 17.11). Together with another protein (Tra-2), Tra stimulates the female-specific splicing of pre-mRNA from yet another gene called doublesex (dsx). This event produces a female-specific Dsx protein, which causes the embryo to develop female characteristics.
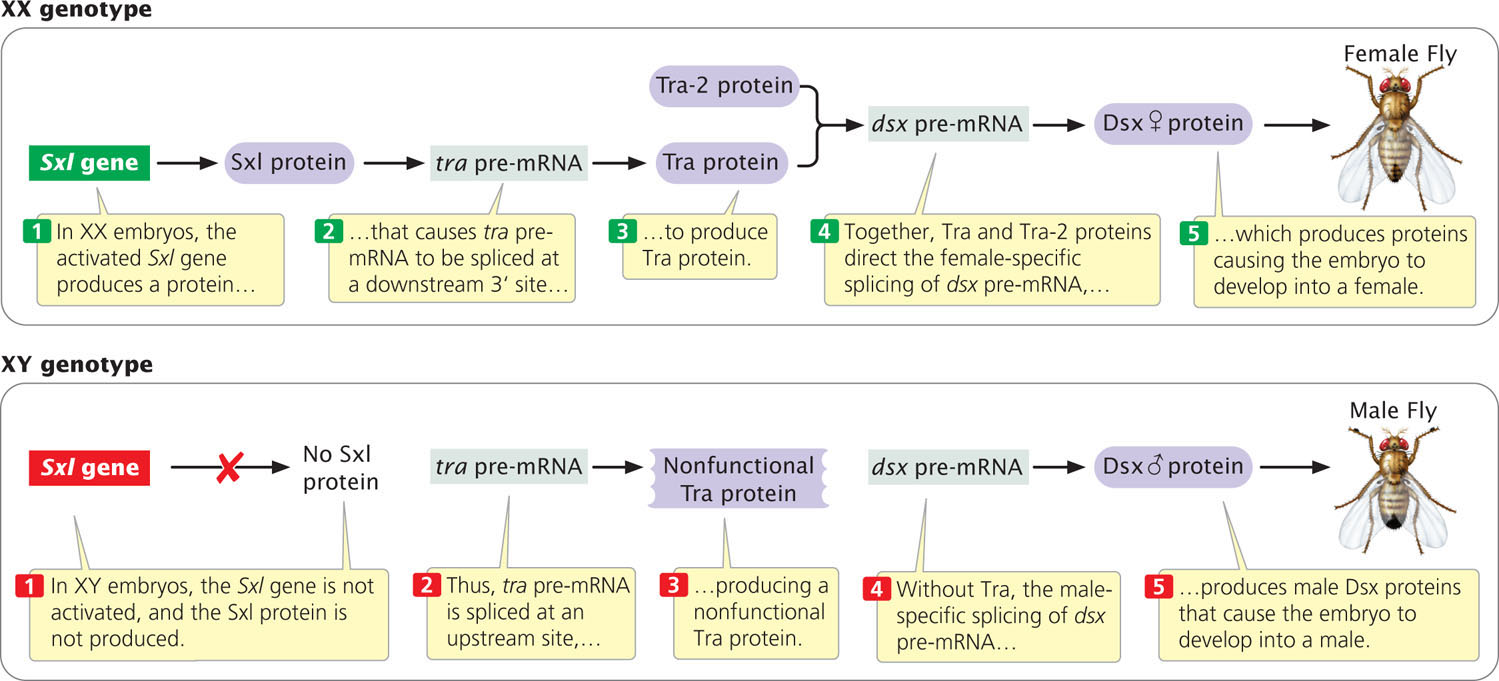
In male embryos, which have a single X chromosome (see Figure 17.11), the promoter that transcribes the Sxl gene in females is inactive, so no Sxl protein is produced. In the absence of Sxl protein, tra pre-mRNA is spliced at a different 3′ splice site to produce a nonfunctional form of Tra protein (Figure 17.12). In turn, the presence of this nonfunctional Tra in males causes dsx pre-mRNAs to be spliced differently from that in females, and a male-specific Dsx protein is produced (see Figure 17.11). This event causes the development of male-specific traits.
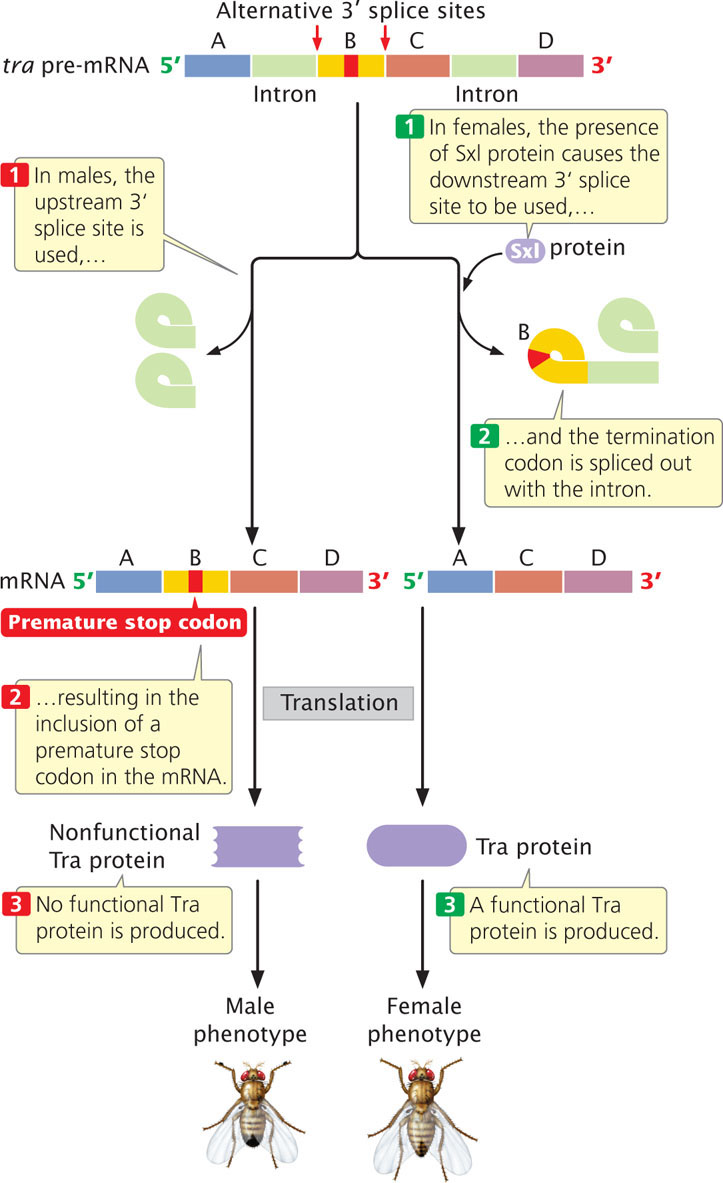
In summary, the Tra, Tra-2, and Sxl proteins regulate alternative splicing that produces male and female phenotypes in Drosophila. Exactly how these proteins regulate alternative splicing is not yet known, but the Sxl protein (produced only in females) possibly blocks the upstream splice site on the tra pre-mRNA. This blockage would force the spliceosome to use the downstream 3′ splice site, which causes the production of Tra protein and eventually results in female traits (see Figure 17.12).  TRY PROBLEM 24
TRY PROBLEM 24
CONCEPTS
Eukaryotic genes can be regulated through the control of mRNA processing. The selection of alternative splice sites leads to the production of different proteins.
The Degradation of RNA
The amount of a protein that is synthesized depends on the amount of corresponding mRNA available for translation. The amount of available mRNA, in turn, depends on both the rate of mRNA synthesis and the rate of mRNA degradation. Eukaryotic mRNAs are generally more stable than bacterial mRNAs, which typically last only a few minutes before being degraded. Nonetheless, there is great variability in the stability of eukaryotic mRNA: some mRNAs persist for only a few minutes; others last for hours, days, or even months. These variations can produce large differences in the amount of protein that is synthesized.
Cellular RNA is degraded by ribonucleases, enzymes that specifically break down RNA. Most eukaryotic cells contain 10 or more types of ribonucleases, and there are several different pathways of mRNA degradation. In one pathway, the 5′ cap is first removed, followed by 5′→3′ removal of nucleotides. A second pathway begins at the 3′ end of the mRNA and removes nucleotides in the 3′→5′ direction. In a third pathway, the mRNA is cleaved at internal sites.
Messenger RNA degradation from the 5′ end is most common and begins with the removal of the 5′ cap. This pathway is usually preceded by the shortening of the poly(A) tail. Poly(A)-binding proteins (PABPs) normally bind to the poly(A) tail and contribute to its stability-enhancing effect. The presence of these proteins at the 3′ end of the mRNA protects the 5′ cap. When the poly(A) tail has been shortened below a critical limit, the 5′ cap is removed, and nucleases then degrade the mRNA by removing nucleotides from the 5′ end. These observations suggest that the 5′ cap and the 3′ poly(A) tail of eukaryotic mRNA physically interact with each other, most likely by the poly(A) tail bending around so that the PABPs make contact with the 5′ cap (see Figure 15.18).
Much of RNA degradation takes place in specialized complexes called P bodies. However, P bodies appear to be more than simply destruction sites for RNA. Evidence suggests that P bodies can temporarily store mRNA molecules, which may later be released and translated. Thus, P bodies help control the expression of genes by regulating which RNA molecules are degraded and which are sequestered for later release. RNA degradation facilitated by small interfering RNAs (siRNAs) also may take place within P bodies (see next section).
Other parts of eukaryotic mRNA, including sequences in the 5′ untranslated region (5′ UTR), the coding region, and the 3′ UTR, also affect mRNA stability. Some short-lived eukaryotic mRNAs have one or more copies of a consensus sequence consisting of 5′-AUUUAUAA-3′, referred to as the AU-rich element, in the 3′ UTR. Messenger RNAs containing AU-rich elements are degraded by a mechanism in which microRNAs take part (see next section).  TRY PROBLEM 26
TRY PROBLEM 26
CONCEPTS
The stability of mRNA influences gene expression by affecting the amount of mRNA available to be translated. The stability of mRNA is affected by the 5′ cap, the poly(A) tail, the 5′ UTR, the coding section, and sequences in 3′ UTR.
 CONCEPT CHECK 4
CONCEPT CHECK 4How does the poly(A) tail affect stability?