23.5 Changes in Chromosome Number and Structure Are Often Associated with Cancer
Most tumors contain cells with chromosome mutations. For many years, geneticists argued about whether these chromosome mutations were the cause or the result of cancer. Some types of tumors are consistently associated with specific chromosome mutations; for example, most cases of chronic myelogenous leukemia are associated with a reciprocal translocation between chromosomes 22 and 9. These types of associations suggest that chromosome mutations contribute to the cause of the cancer. Yet many cancers are not associated with specific types of chromosome abnormalities, and individual gene mutations are now known to contribute to many types of cancer. Nevertheless, chromosome instability is a general feature of cancer cells, causing them to accumulate chromosome mutations, which then affect individual genes that may contribute to the cancer process. Thus, chromosome mutations appear to both cause cancer and result from it.
At least three types of chromosome rearrangements—deletions, inversions, and translocations—are associated with certain types of cancer. Deletions can result in the loss of one or more tumor-suppressor genes. Inversions and translocations contribute to cancer in several ways. First, the chromosomal breakpoints that accompany these mutations can lie within tumor-suppressor genes, disrupting their function and leading to cell proliferation. Second, translocations and inversions can bring together sequences from two different genes, generating a fused protein that stimulates some aspect of the cancer process.
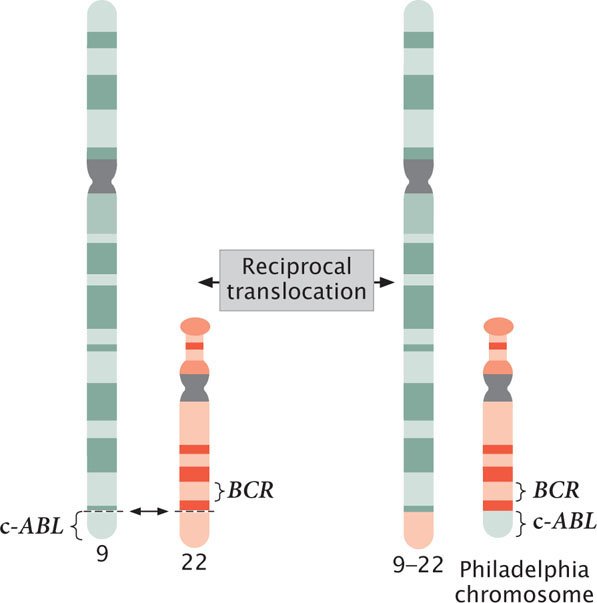
Fusion proteins are seen in most cases of chronic myelogenous leukemia, which affects bone-marrow cells. Most patients with chronic myelogenous leukemia have a reciprocal translocation between the long arm of chromosome 22 and the tip of the long arm of chromosome 9 (Figure 23.11). This translocation produces a shortened chromosome 22, called the Philadelphia chromosome because it was first discovered in Philadelphia. At the end of a normal chromosome 9 is a potential cancer-causing gene called c-ABL. As a result of the translocation, part of the c-ABL gene is fused with the BCR gene from chromosome 22. The protein produced by this BCR–c-ABL fusion gene is much more active than the protein produced by the normal c-ABL gene; the fusion protein stimulates increased, unregulated cell division and eventually leads to leukemia.
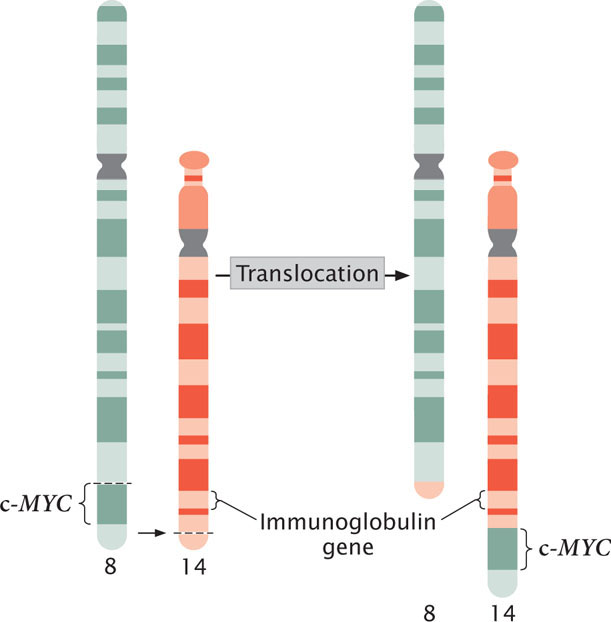
A third mechanism by which chromosome rearrangements can produce cancer is by the transfer of a potential cancer-causing gene to a new location, where it is activated by different regulatory sequences. Burkitt lymphoma is a cancer of the B cells, the lymphocytes that produce antibodies. Many people with Burkitt lymphoma possess a reciprocal translocation between chromosome 8 and chromosome 2, 14, or 22 (Figure 23.12). This translocation relocates a gene called c-MYC from the tip of chromosome 8 to a position on chromosome 2, 14, or 22 that is next to a gene that encodes an immunoglobulin protein. At this new location, c-MYC, a cancer-causing gene, comes under the control of regulatory sequences that normally activate the production of immunoglobulins, and c-MYC is expressed in B cells. The c-MYC protein stimulates the division of the B cells and leads to Burkitt lymphoma.
CONCEPTS
Many tumors contain a variety of types of chromosome mutations. Some tumors are associated with specific deletions, inversions, and translocations. Deletions can eliminate or inactivate genes that control the cell cycle; inversions and translocations can cause breaks in genes that suppress tumors, fuse genes to produce cancer-causing proteins, or move genes to new locations, where they are under the influence of different regulatory sequences.
 CONCEPT CHECK 7
CONCEPT CHECK 7Chronic myelogenous leukemia is usually associated with which type of chromosome rearrangement?
- Duplication.
- Deletion.
- Inversion.
- Translocation.
Most advanced tumors contain cells that exhibit a dramatic variety of chromosome anomalies, including extra chromosomes, missing chromosomes, and chromosome rearrangements (Figure 23.13). Some cancer researchers believe that cancer is initiated when genetic changes take place that cause the genome to become unstable, generating numerous chromosome abnormalities that then alter the expression of oncogenes and tumor-suppressor genes.
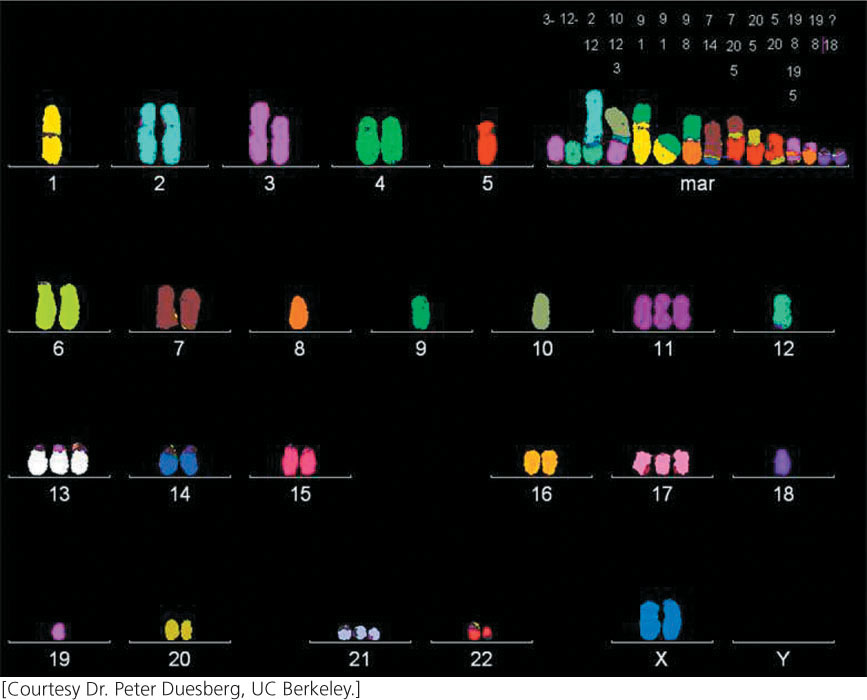
A number of genes that contribute to genomic instability and lead to missing or extra chromosomes (aneuploidy) have now been identified. Aneuploidy in somatic cells usually arises when chromosomes do not segregate properly in mitosis. Normal cells have a spindle-assembly checkpoint that monitors the proper assembly of the mitotic spindle; if chromosomes are not properly attached to the microtubules at metaphase, the onset of anaphase is blocked. Some aneuploid cancer cells contain mutant alleles for genes that encode proteins having roles in this checkpoint; in these cells, anaphase is entered despite the improper assembly of the spindle or lack of it, and chromosome abnormalities result. For example, mutations in RB increase aneuploidy by increasing the expression of a protein called Mad2, which is a critical component of the spindle-assembly checkpoint.
Mutations in genes that encode parts of the spindle apparatus also may contribute to abnormal segregation and lead to chromosome abnormalities. APC is a tumor-suppressor gene that is often mutated in colon-cancer cells. APC has several functions, one of which is to interact with the ends of the microtubules that associate with the kinetochore. Dividing mouse cells that have defective copies of the APC gene give rise to cells with many chromosome defects.
The tumor-suppressor gene p53, in addition to controlling apoptosis, plays a role in the duplication of the centrosome, which is required for proper formation of the spindle and for chromosome segregation. Normally, the centrosome duplicates once per cell cycle. If p53 is mutated or missing, however, the centrosome may undergo extra duplications, resulting in the unequal segregation of chromosomes. In this way, mutation of the p53 gene may generate chromosome mutations that contribute to cancer. The p53 gene is also a tumor-suppressor gene that prevents cell division when the DNA is damaged.  TRY PROBLEM 32
TRY PROBLEM 32