7.4 Physical-Mapping Methods Are Used to Determine the Physical Positions of Genes on Particular Chromosomes
Genetic maps reveal the relative positions of genes on a chromosome on the basis of frequencies of recombination, but they do not provide information that allows us to place groups of linked genes on particular chromosomes. Furthermore, the units of a genetic map do not always precisely correspond to physical distances on the chromosome, because a number of factors other than physical distances between genes (such as the type and sex of the organism) can influence recombination. Because of these limitations, physical-mapping methods that do not rely on recombination frequencies have been developed.
Somatic-Cell Hybridization
One method used for positioning genes on chromosomes is somatic-cell hybridization, which requires the fusion of different types of cells. Most mature somatic (nonsex) cells can undergo only a limited number of divisions and therefore cannot be grown continuously. However, cells that have been altered by viruses or derived from tumors that have lost the normal constraints on cell division will divide indefinitely; this type of cell can be cultured in the laboratory to produce a cell line.
Cells from two different cell lines can be fused by treating them with polyethylene glycol or other agents that alter their plasma membranes. After fusion, the cell possesses two nuclei and is called a heterokaryon. The two nuclei of a heterokaryon eventually also fuse, generating a hybrid cell that contains chromosomes from both cell lines. If human and mouse cells are mixed in the presence of polyethylene glycol, the fusion results in human–mouse somatic-cell hybrids (Figure 7.20). The hybrid cells tend to lose chromosomes as they divide and, for reasons that are not understood, chromosomes from one of the species are lost preferentially. In human-mouse somatic-cell hybrids, the human chromosomes tend to be lost, whereas the mouse chromosomes are retained. Eventually, the chromosome number stabilizes when all but a few of the human chromosomes have been lost. Chromosome loss is random and differs among cell lines. The presence of these “extra” human chromosomes in the mouse genome makes it possible to assign human genes to specific chromosomes.
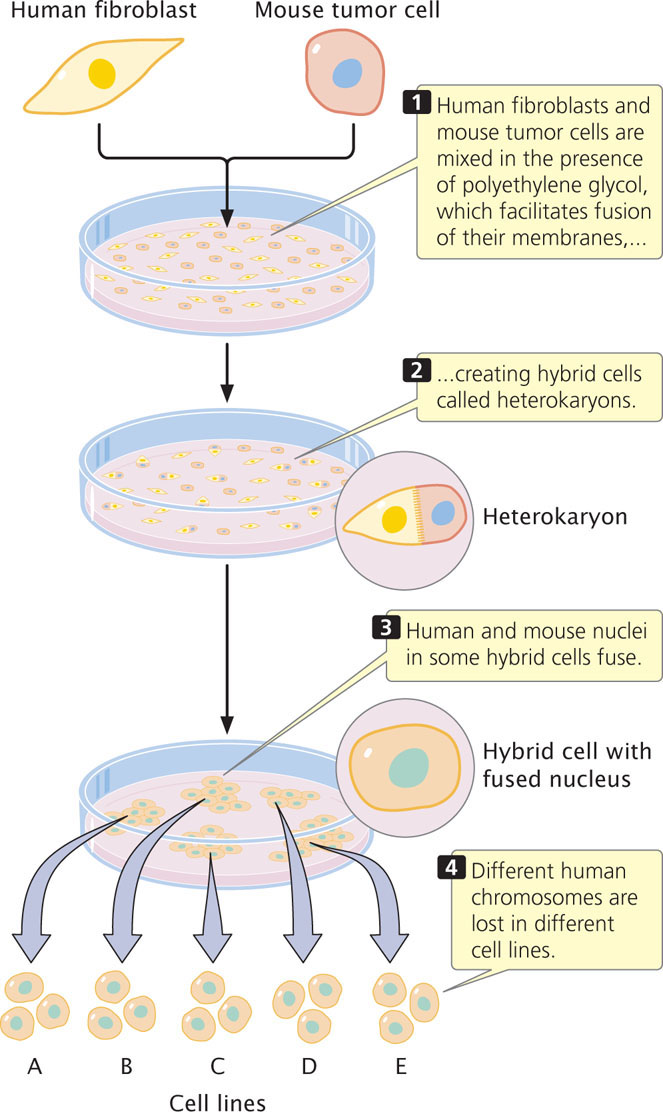
To map genes by using somatic-cell hybridization requires a panel of different hybrid cell lines. Each cell line is examined microscopically and the human chromosomes that it contains are identified. The cell lines of the panel are chosen so that they differ in the human chromosomes that they have retained. For example, one cell line might possess human chromosomes 2, 4, 7, and 8, whereas another might possess chromosomes 4, 19, and 20. Each cell line in the panel is examined for evidence of a particular human gene. The human gene can be detected by looking either for the gene itself (discussed in Chapter 19) or for the protein that it produces. Correlation of the presence of the gene with the presence of specific human chromosomes often allows the gene to be assigned to the correct chromosome. For example, if a gene is detected in both of the aforementioned cell lines, the gene must be on chromosome 4, because it is the only human chromosome common to both cell lines (Figure 7.21).

Sometimes somatic-cell hybridization can be used to position a gene on a specific part of a chromosome. Some hybrid cell lines carry a human chromosome with a mutation such as a deletion or a translocation. If the gene is present in a cell line with the intact chromosome but missing from a line with a chromosome deletion (a mutation in which a part of a chromosome is missing), the gene must be located in the deleted region (Figure 7.22). Similarly, if a gene is usually absent from a chromosome but consistently appears whenever a translocation (a piece of another chromosome that has broken off and attached itself to the chromosome in question) is present, it must be present on the translocated part of the chromosome.

WORKED PROBLEM
A panel of cell lines was created from human–mouse somatic-cell fusions. Each line was examined for the presence of human chromosomes and for the production of human haptoglobin (a protein). The following results were obtained:

On the basis of these results, which human chromosome carries the gene for haptoglobin?
What information is required in your answer to the problem?
The chromosome that carries the gene for haptoglobin.
What information is provided to solve the problem?
 The chromosomes that are present in each cell line (from the table).
The chromosomes that are present in each cell line (from the table). The cell lines that express human haptoglobin (from the table).
The cell lines that express human haptoglobin (from the table).
First, identify the cell lines that are positive for the protein (human haptoglobin) and determine the chromosomes that they have in common. Lines B and C produce human haptoglobin; the only chromosomes that they have in common are chromosomes 1 and 16. Next, examine all the cell lines that possess either chromosomes 1 and 16 and determine whether they produce haptoglobin. Chromosome 1 is found in cell lines A, B, C, and D. If the gene for human haptoglobin were found on chromosome 1, human haptoglobin would be present in all of these cell lines. However, lines A and D do not produce human haptoglobin so the gene cannot be on chromosome 1. Chromosome 16 is found only in cell lines B and C, and only these lines produce human haptoglobin; the gene for human haptoglobin lies on chromosome 16.
For more practice with somatic-cell hybridizations, work Problem 37 at the end of this chapter.
Deletion Mapping
Another method for determining the chromosomal location of a gene is deletion mapping. Special staining methods have been developed that reveal characteristic banding patterns on the chromosomes (see Chapter 9). The absence of one or more of the bands that are normally on a chromosome reveals the presence of a chromosome deletion. Genes can be assigned to regions of chromosomes by studying the association between a gene’s phenotype or product and particular chromosome deletions.
In deletion mapping, an individual that is homozygous for a recessive mutation in the gene of interest is crossed with an individual that is heterozygous for a deletion (Figure 7.23). If the gene of interest is in the region of the chromosome represented by the deletion (the red part of the chromosomes in Figure 7.23), then approximately half of the progeny will display the mutant phenotype (see Figure 7.23a). If the gene is not within the deleted region, then all of the progeny will be wild type (see Figure 7.23b).

Deletion mapping has been used to reveal the chromosomal locations of a number of human genes. For example, Duchenne muscular dystrophy is a disease that causes progressive weakening and degeneration of the muscles. From its X-linked pattern of inheritance, the mutated allele causing this disorder was known to be on the X chromosome, but its precise location was uncertain. Examination of a number of patients with the disease, who also possessed small deletions, allowed researchers to position the gene on a small segment of the short arm of the X chromosome.  TRY PROBLEM 39
TRY PROBLEM 39
Physical Chromosome Mapping Through Molecular Analysis
So far, we have explored methods to indirectly determine the chromosomal location of a gene by looking for gene products or by deletion mapping. Researchers now have the information and technology to actually see where a gene lies. Described in more detail in Chapter 19, in situ hybridization is a method for determining the chromosomal location of a particular gene through molecular analysis. This method requires the creation of a probe for the gene, which is a single-stranded DNA complement to the gene of interest. The probe is radioactive or fluoresces under ultraviolet light so that it can be visualized. The probe binds to the DNA sequence of the gene on the chromosome. The presence of radioactivity or fluorescence from the bound probe reveals the location of the gene on a particular chromosome (Figure 7.24).

In addition to allowing us to see where a gene is located on a chromosome, modern laboratory techniques now allow researchers to identify the precise location of a gene at the nucleotide level. For example, with DNA sequencing (described fully in Chapter 19), physical distances between genes can be determined in numbers of base pairs.
CONCEPTS
Physical-mapping methods determine the physical locations of genes on chromosomes and include deletion mapping, somatic-cell hybridization, in situ hybridization, and direct DNA sequencing.