Changes in Chromatin Structure
One type of gene regulation in eukaryotic cells is accomplished through the modification of chromatin structure. In the nucleus, histone proteins associate to form octamers, around which helical DNA tightly coils to create chromatin (see Figure 8.18). In a general sense, this chromatin structure represses gene expression. For a gene to be transcribed, proteins called transcription factors (see Transcription Factors and Transcriptional Regulator Proteins on the next page) must bind to the DNA. Other regulator proteins and RNA polymerase must also bind to the DNA for transcription to take place. How can these events take place with DNA wrapped tightly around histone proteins? The answer is that, before transcription, chromatin structure changes so that the DNA becomes more accessible to the transcription apparatus.
CHROMATIN REMODELING Some transcription factors and other regulatory proteins alter chromatin structure without altering the chemical structure of the histones directly. These proteins are called chromatin-
One of the best-
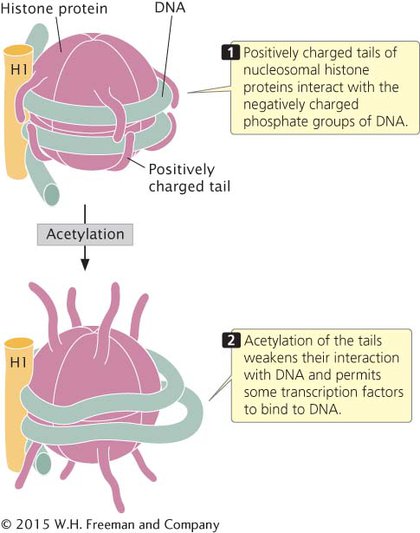
HISTONE MODIFICATION The histones in the octamer core of a nucleosome have two domains: (1) a globular domain that associates with other histones and the DNA and (2) a positively charged tail domain that interacts with the negatively charged phosphate groups on the backbone of DNA. The tails of histone proteins are often modified by the addition or removal of phosphate groups, methyl groups, or acetyl groups. Another modification of histones is ubiquitination, in which small molecules called ubiquitin are added or removed from the histones. All of these modifications have sometimes been collectively called the histone code because they encode information that affects how genes are expressed. The histone code affects gene expression by altering chromatin structure directly or, in some cases, by providing recognition sites for proteins that bind to DNA and then regulate transcription.
METHYLATION OF HISTONES One type of histone modification is the addition of methyl groups to the tails of histone proteins. These modifications can bring about either the activation or the repression of transcription, depending on which histone is modified, which particular amino acids in the tails are methylated, and how many methyl groups are added. Enzymes called histone methyltransferases add methyl groups to specific amino acids (usually lysine or arginine) of histones. Other enzymes, called histone demethylases, remove methyl groups from histones. Many of the enzymes and proteins that modify histones, such as methyltransferases and demethylases, do not bind to specific DNA sequences and must be recruited to specific chromatin sites. Sequence-
A common modification is the addition of three methyl groups to lysine 4 in the tail of the H3 histone protein, abbreviated H3K4me3 (K is the abbreviation for lysine). Histones containing the H3K4me3 modification are frequently found near promoters of transcriptionally active genes. Studies have identified proteins that recognize and bind to H3K4me3, including nucleosome-
ACETYLATION OF HISTONES Another type of histone modification that affects chromatin structure is acetylation, the addition of acetyl groups (CH3CO) to histone. The acetylation of histones usually stimulates transcription. For example, the addition of a single acetyl group to lysine 16 in the tail of the H4 histone prevents the formation of the 30-
THE ACETYLATION OF HISTONES CONTROLS FLOWERING IN ARABIDOPSIS The importance of histone acetylation in gene regulation is demonstrated by the control of flowering in Arabidopsis thaliana, a plant with a number of characteristics that make it an excellent genetic model for plant systems. The time at which flowering takes place is critical to the life of a plant: if flowering is initiated at the wrong time of year, pollinators may not be available to fertilize the flowers or environmental conditions may be unsuitable for the survival and germination of the seeds. Consequently, flowering time in most plants is carefully regulated in response to multiple internal and external cues such as plant size, photoperiod, and temperature.
Among the many genes that control flowering in Arabidopsis is flowering locus C (FLC), which plays an important role in suppressing flowering until after an extended period of cold (a process called vernalization). The FLC gene encodes a regulatory protein that represses the activity of other genes that affect flowering (Figure 12.15). As long as FLC is active, flowering remains suppressed.
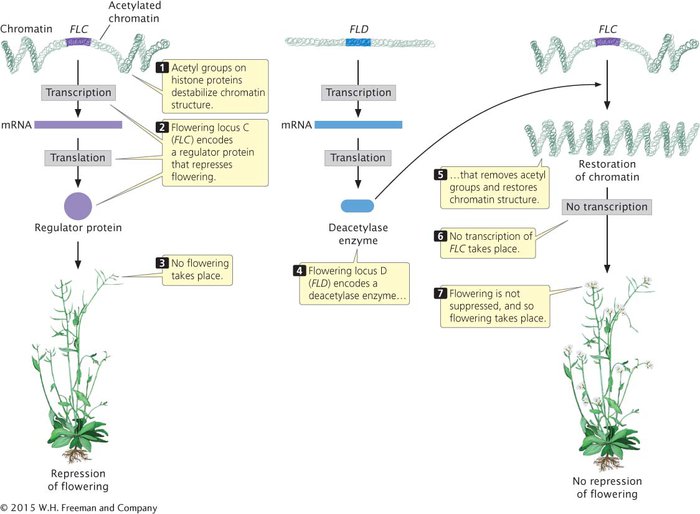
The activity of FLC is controlled by another locus called flowering locus D (FLD), the key role of which is to stimulate flowering by repressing the action of FLC. In essence, flowering is stimulated because FLD represses the repressor. How does FLD repress FLC? FLD encodes a deacetylase enzyme, which removes acetyl groups from histone proteins in the chromatin surrounding FLC (see Figure 12.15). The removal of these acetyl groups alters the chromatin structure and inhibits transcription. The inhibition of transcription prevents FLC from being transcribed and removes its repression on flowering. In short, FLD stimulates flowering in Arabidopsis by deacetylating the chromatin that surrounds FLC, thereby removing its inhibitory effect on flowering.
DNA METHYLATION Another change in chromatin structure associated with transcription is the methylation of cytosine bases, which yields 5-
Evidence indicates that an association exists between DNA methylation and the deacetylation of histones, both of which repress transcription. Certain proteins that bind tightly to methylated sequences form complexes with other proteins that act as histone deacetylases. In other words, methylation appears to attract deacetylases, which remove acetyl groups from the histone tails, stabilizing the nucleosome structure and repressing transcription. The demethylation of DNA allows acetyltransferases to add acetyl groups, disrupting nucleosome structure and permitting transcription. The role of methylation in chromatin structure and epigenetics is discussed further in Section 12.4.
CONCEPTS
Chromatin structure can be altered by chromatin-