Epigenetic Effects
A number of examples of epigenetic effects have been documented. In some cases, the molecular basis of these effects is at least partly understood.
PARAMUTATION Paramutation is defined as an interaction between alleles that leads to a heritable change in expression of one of the alleles. Surprisingly, paramutation produces these differences in phenotype without any alteration in the DNA base sequence of the converted allele. The phenomenon of paramutation has several important features. First, the newly established expression pattern of the converted allele is transmitted to future generations, even when the allele that brought about the alteration is no longer present with it. Second, the altered allele is now able to convert other alleles to the new phenotype. And third, there are no associated DNA sequence differences in the altered alleles. A number of examples of paramutation have now been discovered in different organisms, and geneticists have begun to unravel the molecular mechanisms of this curious phenomenon.
Paramutation occurs in alleles at the b1 locus in corn, which is involved in determining pigmentation. The b1 locus helps to determine the amount of purple anthocyanin that a corn plant produces. The locus actually encodes a transcription factor that regulates genes involved in pigment production. Plants homozygous for the B-
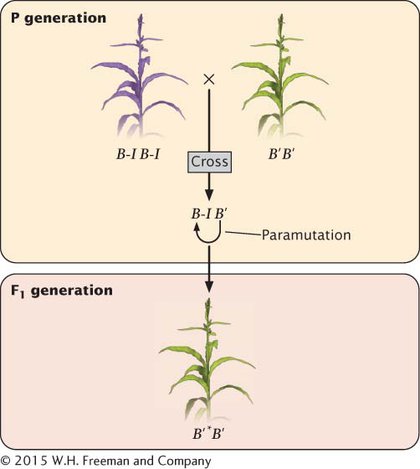
In plants that are heterozygous B-
EPIGENETIC CHANGES INDUCED BY MATERNAL BEHAVIOR A fascinating example of epigenetics is seen in the long-
To demonstrate the effect of altered chromatin structure on the stress responses of the offspring, researchers infused the brains of young rats with a deacetylase inhibitor, which prevents the removal of acetyl groups from the histone proteins. After infusion of the deacetylase inhibitor, differences in DNA methylation and histone acetylation associated with grooming behavior disappeared, as did the difference in responses to fear and stress in the offspring when they reached adulthood. This demonstrates that the mother rat’s licking and grooming behavior brings about epigenetic changes in the offspring’s chromatin, which causes long- TRY PROBLEM 38
TRY PROBLEM 38
EPIGENETIC EFFECTS OF ENVIRONMENTAL CHEMICALS Because some chemicals are capable of modifying chromatin structure, researchers have looked for long-
In one study, researchers found that the exposure of female rats to vinclozolin led to reduced sperm production not only in their male offspring (when they reached puberty), but also in several subsequent generations. Increased DNA methylation was seen in sperm of the males that were exposed to vinclozolin, and these patterns of methylation were inherited. This study and others have raised concerns that, through epigenetic changes, environmental exposure to some chemicals might have effects on the health of future generations.
EPIGENETIC EFFECTS IN MONOZYGOTIC TWINS Monozygotic (identical) twins develop from a single egg fertilized by a single sperm that divides and gives rise to two zygotes. Monozygotic twins are genetically identical, in the sense that they possess identical DNA sequences, but they often differ somewhat in appearance, health, and behavior. The nature of these differences in the phenotypes of identical twins is not well understood, but recent evidence suggests that at least some of these differences may be due to epigenetic changes. In one study, Mario Fraga at the Spanish National Cancer Center and his colleagues examined 80 pairs of identical twins and compared the degree and location of their DNA methylation and histone acetylation. They found that DNA methylation and histone acetylation in pairs of identical twins were similar early in life, but that older twin pairs had remarkable differences in their overall content and distribution of DNA methylation and histone acetylation. Furthermore, these differences affected gene expression in the twins. This research suggests that identical twins do differ epigenetically and that phenotypic differences between them may be caused by differential gene expression.