The lac Operon of Escherichia coli
As we saw at the start of this chapter, François Jacob and Jacques Monod first described the “operon model” of the genetic control of lactose metabolism in E. coli. Their work and subsequent research on the genetics of lactose metabolism established the operon as the basic unit of transcriptional control in bacteria. At the time, no methods were available for determining nucleotide sequences; Jacob and Monod deduced the structure of the operon genetically by analyzing the interactions of mutations that interfered with the normal regulation of lactose metabolism. We will examine the effects of some of these mutations after seeing how the lac operon regulates lactose metabolism.
LACTOSE METABOLISM Lactose is one of the major carbohydrates found in milk; it can be metabolized by E. coli bacteria that reside in the mammalian gut. Lactose does not easily diffuse across the E. coli cell membrane and must be actively transported into the cell by the protein permease (Figure 12.6). To use lactose as an energy source, E. coli must first break it into glucose and galactose, a reaction catalyzed by the enzyme β-galactosidase. This enzyme can also convert lactose into allolactose, a compound that plays an important role in regulating lactose metabolism. A third enzyme, thiogalactoside transacetylase, is also produced by the lac operon, but its function in lactose metabolism is not yet clear. One possible function is detoxification, preventing the accumulation of thiogalactosides that are transported into the cell along with lactose by lactose permease.
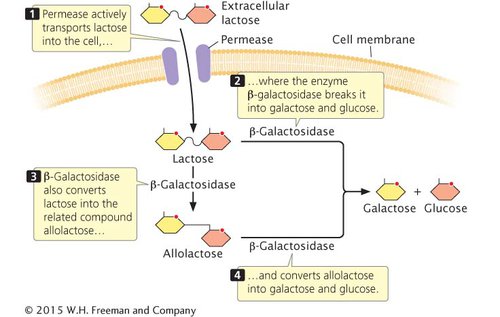
REGULATION OF THE lac OPERON The lac operon of E. coli is an example of a negative inducible operon. The proteins β-galactosidase, permease, and transacetylase are encoded by adjacent structural genes in the lac operon (Figure 12.7a) and have a common promoter (lacP in Figure 12.7a). β-Galactosidase is encoded by the lacZ gene, permease by the lacY gene, and transacetylase by the lacA gene. When lactose is absent from the medium in which E. coli grows, few molecules of each protein are produced. If lactose is added to the medium and glucose is absent, the rate of synthesis of all three proteins simultaneously increases about a thousandfold within 2 to 3 minutes. This boost in protein synthesis, which results from the simultaneous transcription of lacZ, lacY, and lacA, exemplifies coordinate induction, stimulation of the simultaneous synthesis of several proteins by a specific molecule, the inducer (Figure 12.7b).
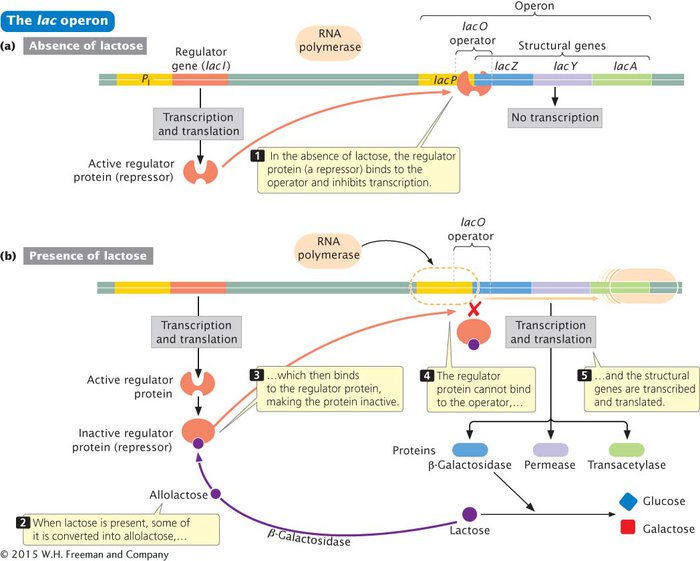
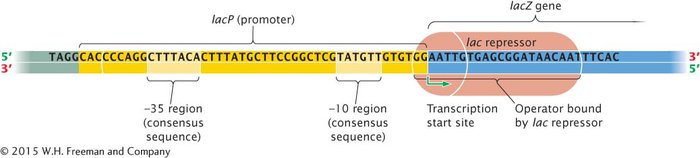
Although lactose might appear to be the inducer in this case, allolactose is actually responsible for induction. Upstream of lacP is a regulator gene, lacI, which has its own promoter (PI). The lacI gene is transcribed into a short mRNA that is translated into a repressor. The repressor consists of four identical polypeptides and has two types of binding sites: one type of site binds to allolactose and the other binds to DNA. In the absence of lactose (and, therefore, allolactose), the repressor binds to the lac operator lacO (see Figure 12.7a). The location of the operator relative to the promoter and the lacZ gene is shown in Figure 12.8.
RNA polymerase binds to the promoter and moves down the DNA molecule, transcribing the structural genes. When the repressor is bound to the operator, the binding of RNA polymerase is blocked, and transcription is prevented. When lactose is present, some of it is converted into allolactose, which binds to the repressor and causes the repressor to be released from the DNA. In the presence of lactose, then, the repressor is inactivated, the binding of RNA polymerase is no longer blocked, the transcription of lacZ, lacY, and lacA takes place, and the lac operon proteins are produced.
Have you spotted the flaw in the explanation just given for the induction of the lac operon proteins? You might recall that permease is required to transport lactose into the cell. If the lac operon is repressed and no permease is being produced, how does lactose get into the cell to inactivate the repressor and turn on transcription? Furthermore, the inducer is actually allolactose, which must be produced from lactose by β-galactosidase. If β-galactosidase production is repressed, how can lactose metabolism be induced?
The answer is that repression never completely shuts down transcription of the lac operon. Even with active repressor bound to the operator, there is a low level of transcription, and a few molecules of β-galactosidase, permease, and transacetylase are synthesized. When lactose appears in the medium, the permease that is present transports a small amount of lactose into the cell. There, the few molecules of β-galactosidase that are present convert some of the lactose into allolactose, which then induces transcription.
CONCEPTS
The lac operon of E. coli controls the transcription of three genes needed in lactose metabolism: the lacZ gene, which encodes β-galactosidase; the lacY gene, which encodes permease; and the lacA gene, which encodes thiogalactoside transacetylase. The lac operon is a negative inducible operon: a regulator gene produces a repressor that binds to the operator and prevents the transcription of the structural genes. The presence of allolactose inactivates the repressor and allows the transcription of the lac operon.
 CONCEPT CHECK 5
CONCEPT CHECK 5
In the presence of allolactose, the lac operon repressor
binds to the operator.
binds to the promoter.
cannot bind to the operator.
binds to the regulator gene.
c