Next-Generation Sequencing Technologies
Newer methods, called next-generation sequencing technologies, have made sequencing hundreds of times faster and less expensive that the traditional Sanger sequencing method. Most next-generation sequencing technologies do sequencing in parallel, which means that hundreds of thousands or even millions of DNA fragments can be sequenced simultaneously, allowing, for example, a human genome to be sequenced in days instead of years.
PYROSEQUENCING One next-generation sequencing technique, called pyrosequencing, is based on DNA synthesis: nucleotides are added one at a time in the order specified by template DNA. The addition of a particular nucleotide is detected with a flash of light, which is generated as the nucleotide is added.
To carry out pyrosequencing, DNA to be sequenced is first fragmented. An adaptor, consisting of a short string of nucleotides, is added to each fragment (Figure 14.16a). The adaptor provides a known sequence to prime a PCR reaction. The DNA fragments are then made single stranded. In one version of pyrosequencing, each fragment is then attached to a separate microscopic bead and surrounded by a droplet of solution containing the reagents for PCR (Figure 14.16b). The bead is used to hold the DNA and later, to deposit it on a plate for the sequencing reaction. Within the droplet, the fragment is amplified by PCR, and the copies of DNA remain attached to the bead. After amplification, the beads are mixed with DNA polymerase and deposited on plates containing more than a million wells (small holes), with each bead going into a separate well.
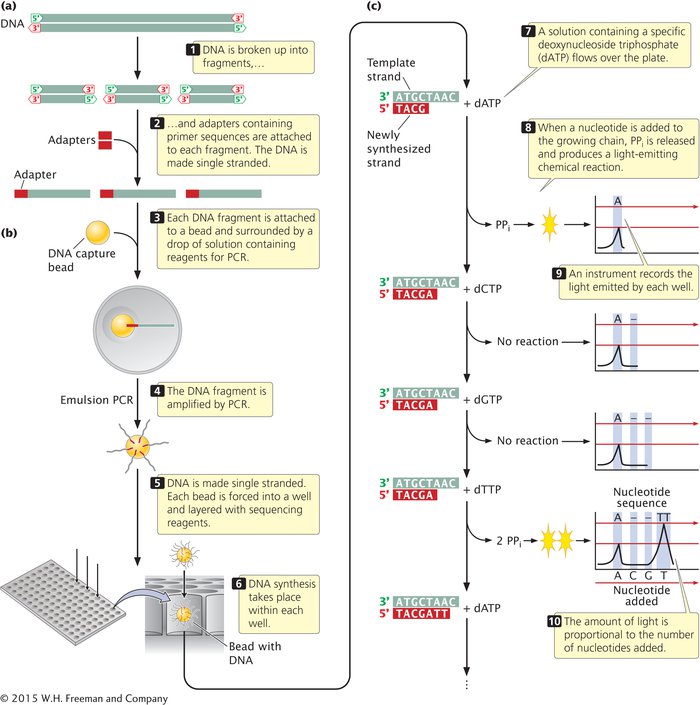
14.16 Next-generation sequencing methods are able to simultaneously determine the sequences of hundreds of thousands or millions of DNA fragments. Pyrosequencing is illustrated here.
Page 388
The sequencing reaction that takes place in each well is based on DNA synthesis. Recall from Chapter 9 that deoxyribonucleoside triphosphate—the substrate for DNA synthesis—consists of a deoxyribose sugar attached to a base and three phosphate groups. In the process of DNA synthesis, two phosphate groups (pyrophosphate, or PPi) are cleaved off, and the resulting nucleotide is attached to the 3′ end of the growing DNA chain. A solution containing one particular type of dNTP—say, dATP—is passed across the wells (Figure 14.16c). If the template within a particular well specifies an adenine nucleotide in the next position of the growing chain, then pyrophosphate is cleaved from the dATP, and the adenine nucleotide is added. A chemical reaction uses the pyrophosphate produced in the reaction to generate a flash of light, which is measured by an optical detector. The amount of light emitted in each well is proportional to the number of nucleotides added: if the template in a well specifies three successive adenine nucleotides, then three nucleotides are added, and three times more light is emitted than if a single A is added. If the position in the template specifies a base other than adenine, no nucleotide is added, no pyrophosphate is produced, and no light is emitted.
As mentioned, the first solution passed over the plate contains adenosine triphosphate and allows adenine nucleotides to be added to the template. Each well with a template that specifies adenine in the next position will generate a flash of light. Then a solution with a different type of dNTP—say, dGTP—is passed across the wells. Any fragment that specifies a G in the next position of its growing chain will add a guanine nucleotide and emit a flash of light. The nucleotide triphosphates are passed across the wells in a predetermined order, and the light emitted by each well is measured. In this way, hundreds of thousands or millions of fragments of DNA are sequenced simultaneously on the basis of the order in which nucleotides are added to the 3′ end of the growing chain.
ILLUMINA SEQUENCING Several other forms of next-generation sequencing are widely used. Illumina sequencing (Figure 14.17) employs a technology similar to that of the Sanger dideoxy method. Special nucleotides are used that have a fluorescent tag attached, with a different-colored tag for each type of nucleotide. Each nucleotide also has a chemical group (a terminator) that, once incorporated into the growing DNA chain, prevents the incorporation of any additional nucleotides, as ddNTPs do in Sanger sequencing. In this case, however, the terminator is reversible—it can be chemically removed.
14.17 Illumina sequencing is widely used in next-generation sequencing technology. The image shows sequencing underway on the surface of a flow cell in an Illumina next-generation DNA sequencer. The color of the each dot indicates the type of nucleotide (A, T, G, or C) added to the end of a specific DNA fragment.
[Image reproduced with permission of Illumina, Inc.]
To carry out sequencing, the DNA is first fragmented into millions of short overlapping fragments. The fragments are attached to a slide and then amplified, creating clusters of up to a thousand copies of each fragment in close proximity on the slide. The fragments are then denatured, and a solution of primers, DNA polymerase, and the special nucleotides is added. The primer attaches to each DNA template, and the first nucleotide is incorporated into the newly synthesized strand. The solution is washed away, and the tag on the incorporated nucleotide is excited with a laser, which causes it to fluoresce. As mentioned above, each type of nucleotide (A, T, G, or C) has a different-colored fluorescent tag, so the color of the light produced reveals the type of the nucleotide just added. The terminator and the fluorescent tag are then chemically removed, and the process is repeated. As the nucleotides are added one at a time, the sequence is read as a series of flashes of colored light from each cluster of DNA. Hundreds of thousands of DNA clusters, each consisting of copies of a different DNA fragment, are sequenced simultaneously, allowing large amounts of DNA to be sequenced in a short time.
Most next-generation sequencing techniques read shorter DNA fragments than the Sanger sequencing method, but because hundreds of thousands or millions of fragments are sequenced simultaneously, these methods are much faster than traditional Sanger sequencing technology. But these techniques only determine the sequences of the short DNA fragments; they do not, by themselves, allow the sequences of these fragments to be reassembled into the sequence of the entire original piece of DNA. How sequences of small fragments are reassembled into a continuous sequence of DNA is explained in Chapter 15.
Page 389
THIRD-GENERATION SEQUENCING TECHNOLOGY Even more advanced and rapid sequencing methods, typically called third-generation sequencing methods, are being developed. Nanopore sequencing, for example, can determine the sequence of a single molecule of DNA. In this method, a single strand of DNA is passed through a tiny hole—a nanopore—in a membrane. As the molecule passes, one nucleotide at a time, through the nanopore, it disrupts an electrical current in the membrane, and the nature of the disruption is affected by the shape of the nucleotide passing through the nanopore. Each of the four types of nucleotides of DNA causes a characteristic disruption, so the sequence of DNA can be read by analyzing the membrane current as the strand passes through the nanopore. Hundreds of thousands of nanopores can be created on a single chip, so that many DNA fragments can be read simultaneously. One of the goals of third-generation sequencing technology is to develop a method that can sequence an entire human genome for less than $1000.
CONCEPTS
New next- and third-generation sequencing methods can sequence many DNA fragments simultaneously, providing much faster and less expensive determination of DNA base sequences.

