Cloning Genes
Many recombinant DNA methods require numerous copies of a specific DNA fragment. One way to amplify (make multiple copies of) a specific piece of DNA is to place the fragment in a bacterial cell and allow the cell to replicate the DNA. This procedure is termed gene cloning because identical copies (clones) of the original piece of DNA are produced.
A cloning vector is a stable, replicating DNA molecule into which a foreign DNA fragment can be inserted for introduction into a cell. An effective cloning vector has three important characteristics (Figure 14.4): (1) an origin of replication, which ensures that the vector is replicated within the cell; (2) selectable markers, which enable any cells containing the vector to be selected or identified; and (3) one or more unique restriction sites into which a DNA fragment can be inserted. The vector must have only one (unique) restriction site for each restriction enzyme used; if a vector is cut at multiple restriction sites, several pieces of DNA are generated, and getting these pieces back together in the correct order is possible, but extremely difficult.
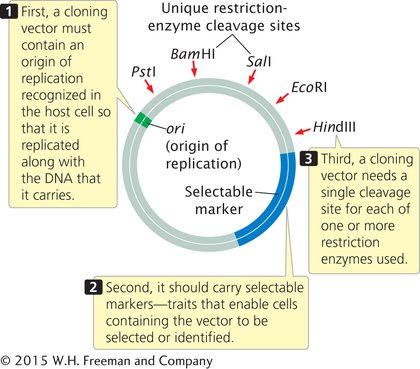
PLASMID VECTORS Plasmids, circular DNA molecules that exist naturally in bacteria (see Chapter 7), are commonly used as vectors for cloning DNA fragments in bacteria. They contain origins of replication and are therefore able to replicate independently of the bacterial chromosome. The plasmids typically used in cloning have been artificially constructed from larger, naturally occurring bacterial plasmids and have recognition sequences for multiple enzymes, an origin of replication, and selectable markers.
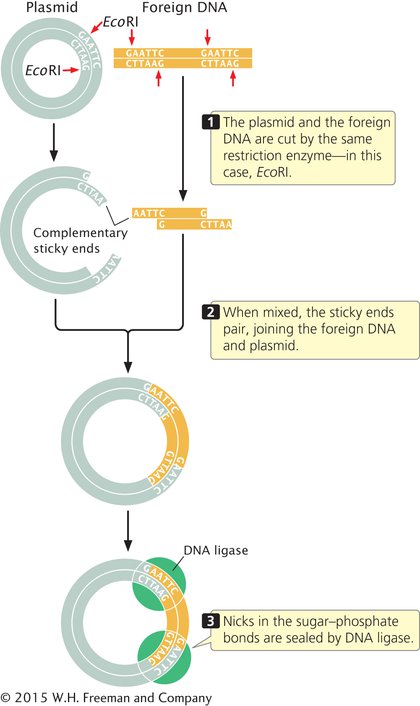
The easiest method for inserting a particular DNA sequence into a plasmid vector is to cut the foreign DNA (containing a DNA fragment of interest) and the plasmid with the same restriction enzyme (Figure 14.5). If the restriction enzyme makes staggered cuts in the DNA, complementary sticky ends are produced on the foreign DNA and the plasmid. The DNA and plasmid are then mixed together; some of the foreign DNA fragments will pair with the cut ends of the plasmid. DNA ligase is used to seal the nicks in the sugar–phosphate backbone, creating a recombinant plasmid that contains the foreign DNA fragment. You can learn more about plasmid cloning by viewing Animation 14.1.
TRANSFORMATION Once a DNA fragment of interest has been placed inside a plasmid, the plasmid must be introduced into bacterial cells. This task is usually accomplished by transformation, the mechanism by which bacterial cells take up DNA from the external environment (see Chapter 7). Some types of cells undergo transformation naturally; others must be treated chemically or physically before they will undergo transformation. Inside the cells, the plasmids replicate and multiply, and the cells themselves multiply, producing many copies of the introduced gene.
SCREENING CELLS FOR RECOMBINANT PLASMIDS Cells bearing recombinant plasmids can be detected by using the selectable markers on the plasmid. Genes that confer resistance to an antibiotic are commonly used as selectable markers; any cell that contains such a plasmid will be able to live in the presence of the antibiotic, which normally kills bacterial cells.
A common way to screen cells for the presence of a recombinant plasmid is to use a plasmid with a copy of the lacZ gene as a vector (Figure 14.6). The lacZ gene contains a series of unique restriction sites at which a piece of DNA can be inserted. In the absence of an inserted fragment, the lacZ gene is active and produces β-
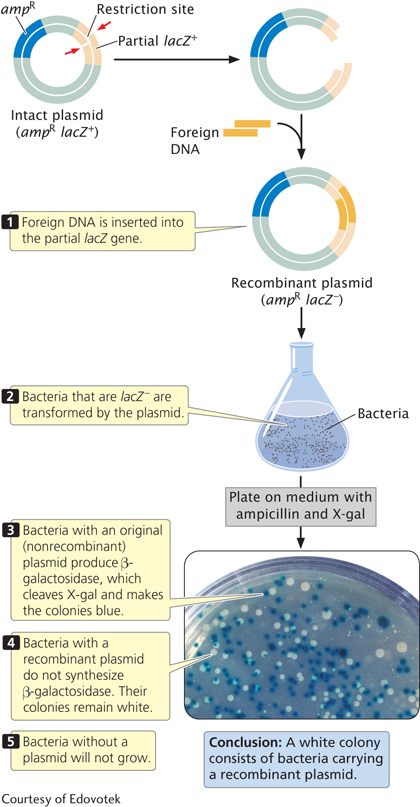
Bacteria that are lacZ− are exposed to the plasmids and then plated on medium that contains ampicillin. Only cells that have been successfully transformed and thus contain a plasmid with the ampicillin-
Plasmids make ideal cloning vectors, but can hold only DNA fragments less than about 15 kb in size. When large DNA fragments are inserted into a plasmid vector, the plasmid becomes unstable and tends to spontaneously lose DNA.
OTHER CLONING VECTORS A number of other vectors have been developed for cloning larger pieces of DNA in bacteria. For example, bacteriophage λ (lambda), which infects E. coli, can be used to clone up to 23,000 bp of foreign DNA; it transfers DNA into bacterial cells with high efficiency. Cosmids are plasmids that are packaged into empty viral protein coats and transferred to bacteria by viral infection. They can carry more than twice as much foreign DNA as can a phage vector. Bacterial artificial chromosomes (BACs) are vectors originally constructed from the F plasmid (a special plasmid that controls mating and the transfer of genetic material in some bacteria; see Chapter 7) and can hold very large fragments of DNA that can be as long as 300,000 bp. Table 14.2 compares the properties of plasmids, phage λ, cosmids, and BACs.
| Cloning vector | Size of DNA that can be cloned | Method of propagation | Introduction to bacteria |
|---|---|---|---|
| Plasmid | As large as 15 kb | Plasmid replication | Transformation |
| Phage λ | As large as 23 kb | Phage reproduction | Phage infection |
| Cosmid | As large as 44 kb | Plasmid reproduction | Phage infection |
| Bacterial artificial chromosome | As large as 300 kb | Plasmid reproduction | Electroporation |
|
Note: 1 kb = 1000 bp. Electroporation is the use of electrical pulses to increase the permeability of a membrane. |
|||
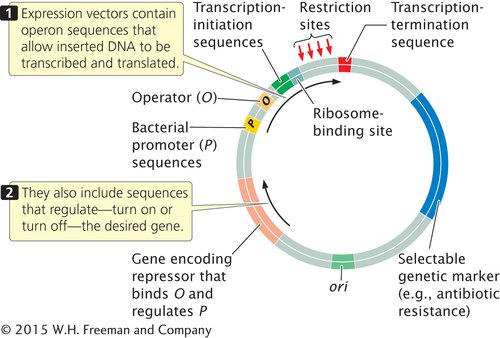
Sometimes the goal in gene cloning is not just to replicate the gene, but also to produce the protein that it encodes. To ensure transcription and translation, a foreign gene is usually inserted into an expression vector, which, in addition to the usual origin of replication, unique restriction sites, and selectable markers, contains sequences required for transcription and translation in bacterial cells (Figure 14.7).
Although manipulating genes in bacteria is simple and efficient, the goal may be to transfer a gene into eukaryotic cells. For example, it might be desirable to transfer a gene conferring herbicide resistance into a crop plant or to transfer a gene for clotting factor into a person suffering from hemophilia. Many eukaryotic proteins are modified after translation (e.g., sugar groups may be added). Such modifications are essential for proper function, but bacteria do not have the ability to carry them out; thus, a functional protein can be produced only in a eukaryotic cell. A number of cloning vectors have been developed that allow the insertion of genes into eukaryotic cells. For example, the Ti plasmid, a large plasmid from the soil bacterium Agrobacterium tumefaciens, has been genetically engineered to transfer genes to plants, including genes that confer economically significant attributes such as resistance to herbicides, plant viruses, and insect pests.  TRY PROBLEM 21
TRY PROBLEM 21
CONCEPTS
DNA fragments can be inserted into cloning vectors, stable pieces of DNA that will replicate within a cell. A cloning vector must have an origin of replication, one or more unique restriction sites, and selectable markers. An expression vector contains additional sequences that allow a cloned gene to be transcribed and translated. Special cloning vectors have been developed for introducing genes into eukaryotic cells.
 CONCEPT CHECK 3
CONCEPT CHECK 3
How is a gene inserted into a plasmid cloning vector?
The gene and plasmid are cut with the same restriction enzyme and mixed together. DNA ligase is used to seal nicks in the sugar–phosphate backbone.