Genes That Control the Cell Cycle
The cell cycle is the normal process by which cells undergo growth and division. Normally, progression through the cell cycle is tightly regulated so that cells divide only when additional cells are needed, when all the components necessary for division are present, and when the DNA has been replicated without damage. Sometimes, however, errors arise in one or more of the components that regulate the cell cycle. These errors often cause cells to divide at inappropriate times or rates, leading to cancer. Indeed, many proto-oncogenes and tumor-suppressor genes function normally by helping to control the cell cycle. Before considering how errors in this system contribute to cancer, we must first understand how the cell cycle is usually regulated.
CONTROL OF THE CELL CYCLE As discussed in Chapter 2, the cell cycle consists of the period from one cell division to the next. Cells that are actively dividing pass through the G1, S, and G2 phases of interphase and then move directly into the M phase, in which cell division takes place. Nondividing cells pass from G1 into the G0 phase, in which they are functional but not actively growing or dividing. Progression from one phase of the cell cycle to another is influenced by a number of internal and external signals and is regulated at key points in the cycle, called checkpoints.
Page 435
For many years, the biochemical events that control the progression of cells through the cell cycle were completely unknown, but research findings have now revealed many of the details of this process. Key events of the cell cycle are controlled by cyclin-dependent kinases (CDKs), which are enzymes that phosphorylate (add phosphate groups to) other proteins. In some cases, phosphorylation activates the other protein, and in others, it inactivates the other protein. As their name implies, CDKs are functional only when associated with another type of protein, called a cyclin. The levels of cyclins oscillate over the course of the cell cycle; when bound to a CDK, a cyclin specifies which proteins the CDK will phosphorylate. Each cyclin appears at a specific point in the cell cycle, usually because its synthesis and destruction are regulated by another cyclin. Cyclins and CDKs are called by different names in different organisms; here, we will use the terms applied to these molecules in mammals.
Let’s look at the G1-to-S transition. As stated in Chapter 2, progression through the cell cycle is regulated at several checkpoints, which ensure that all cellular components are present and in good working order before the cell proceeds to the next phase. The G1/S checkpoint is at the end of G1, just before the cell enters the S phase and replicates its DNA. The cell is prevented from passing through the G1/S checkpoint by a molecule called the retinoblastoma (RB) protein (Figure 16.7), which binds to another molecule called E2F and keeps it inactive. During G1, cyclin D and cyclin E continuously increase in concentration and combine with their associated CDKs. Cyclin-D–CDK and cyclin-E–CDK both phosphorylate molecules of RB. By late in G1, phosphorylation of RB is completed, which inactivates RB. Without the inhibitory effects of RB, E2F is released. The E2F protein stimulates the transcription of genes that produce enzymes necessary for replication of the DNA, and the cell moves into the S phase of the cell cycle.
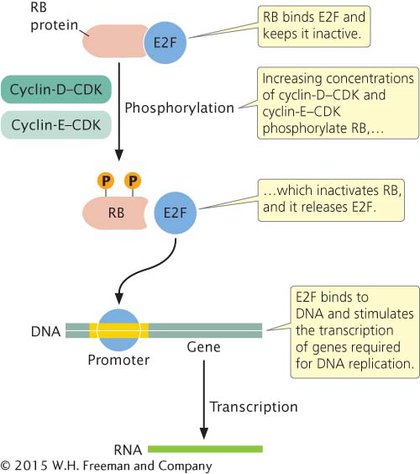
16.7 The RB protein helps control the progression through the G1/S checkpoint by binding transcription factor E2F.
Another important checkpoint controls the G2-to-M transition. This checkpoint is also regulated by CDKs and cyclins. Other checkpoints control the assembly of the mitotic spindle apparatus and the cell’s exit from mitosis.
MUTATIONS IN CELL-CYCLE CONTROLS AND CANCER Many cancers are caused by defects in the cell cycle’s regulatory machinery. For example, mutations in the gene that encodes the RB protein—which normally holds the cell in G1 until the DNA is ready to be replicated—are associated with many cancers, including retinoblastoma. When the RB gene is mutated, cells pass through the G1/S checkpoint without the normal controls that prevent cell proliferation. Overexpression of the gene that encodes cyclin D (which stimulates the passage of cells through the G1/S checkpoint) takes place in about 50% of all breast cancers as well as in some cases of esophageal and skin cancer. Likewise, the tumor-suppressor gene p53, which is mutated in about 75% of all colon cancers, regulates a potent inhibitor of CDK activity.
Some proto-oncogenes and tumor-suppressor genes have roles in apoptosis, a process of programmed cell death in which a cell’s DNA is degraded, its nucleus and cytoplasm shrink, and the cell undergoes phagocytosis by other cells without the leakage of its contents. Cells have the ability to assess themselves, and if they are abnormal or damaged, they normally undergo apoptosis. Many cancer cells have chromosome mutations, DNA damage, or other cellular anomalies that would normally stimulate apoptosis and prevent their proliferation. Often, these cells have mutations in genes that regulate apoptosis, and therefore they do not undergo programmed cell death. The ability of a cell to initiate apoptosis in response to DNA damage, for example, depends on p53, which is inactive in many human cancers.
CONCEPTS
Progression through the cell cycle is controlled at checkpoints, which are regulated by interactions between cyclins and cyclin-dependent kinases. Genes that control the cell cycle are frequently mutated in cancer cells.
