Conjugation
In 1946, Joshua Lederberg and Edward Tatum demonstrated that bacteria can transfer and recombine genetic information, paving the way for the use of bacteria in genetic studies. In the course of their research, Lederberg and Tatum studied auxotrophic strains of E. coli. The Y10 strain required the amino acids threonine (and was genotypically thr−)
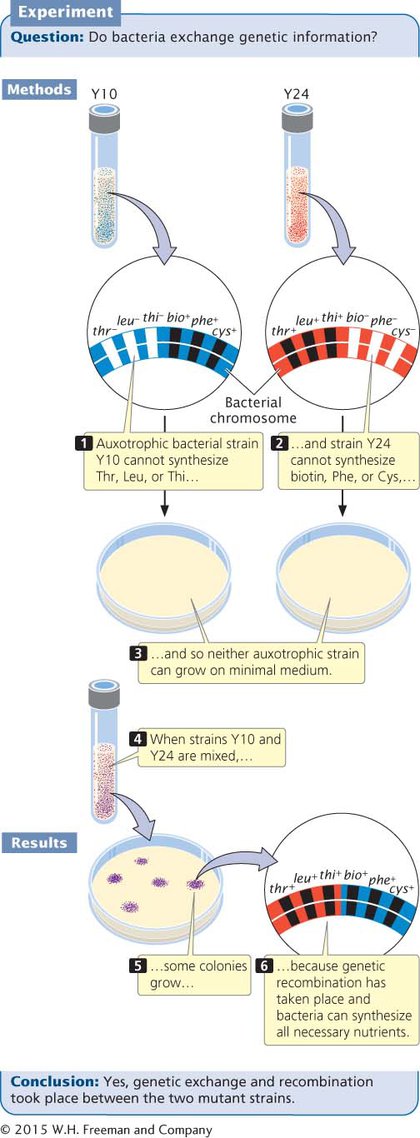
Alone, neither Y10 nor Y24 grew on minimal medium: each strain required nutrients that were absent. Strain Y10 was unable to grow because it required threonine, leucine, and thiamine, which were absent in the minimal medium; strain Y24 was unable to grow because it required biotin, phenylalanine, and cysteine, which also were absent from the minimal medium. When Lederberg and Tatum mixed the two strains, however, a few colonies did grow on the minimal medium. These prototrophic bacteria must have had genotype thr+ leu+ thi+ bio+ phe+ cys+. Where had they come from?
If mutations were responsible for the prototrophic colonies, then some colonies should also have grown on the plates containing Y10 or Y24 alone, but no bacteria grew on those plates. Multiple simultaneous mutations (thr− → thr+, leu− → leu+, and thi− → thi+ in strain Y10 or bio− → bio+, phe− → phe+, and cys− → cys+ in strain Y24) would have been required for either strain to become prototrophic by mutation, which was very improbable. Lederberg and Tatum concluded that some type of genetic transfer and recombination had taken place:
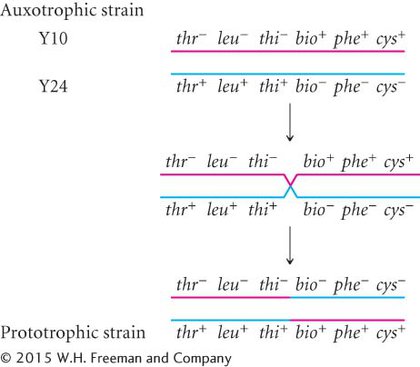
What they did not know was how it had taken place.
To study this problem, Bernard Davis constructed a U-
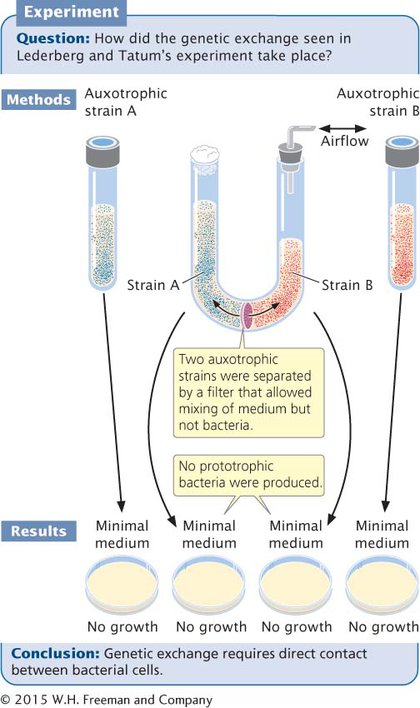
F+ AND F− CELLS In most bacteria, conjugation depends on a fertility (F) factor that is present in the donor cell and absent in the recipient cell. Cells that contain the F factor are referred to as F+, and cells lacking the F factor are F−.
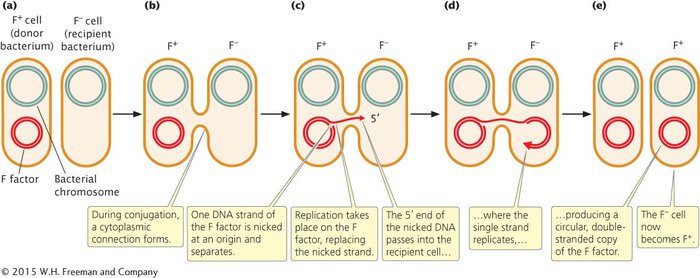
The F factor is an episome that contains an origin of replication and a number of genes required for conjugation (see Figure 7.6). For example, some of these genes encode sex pili (singular, pilus), slender extensions of the cell membrane. A cell containing the F factor produces sex pili, one of which makes contact with a receptor on an F− cell (Figure 7.10) and pulls the two cells together. DNA is then transferred from the F+ cell to the F− cell. Conjugation can take place only between a cell that possesses the F factor and a cell that lacks the F factor.
In most cases, the only genes transferred during conjugation between an F+ and F− cell are those on the F factor (Figure 7.11a and b). Transfer is initiated when one of the DNA strands on the F factor is nicked at an origin of transfer (oriT). One end of the nicked DNA separates from the circular F factor and passes into the recipient cell (Figure 7.11c). Replication takes place on the nicked strand, proceeding around the F factor in the F+ cell and replacing the transferred strand (Figure 7.11d). Because the F factor in the donor cell is always nicked at the oriT site, this site always enters the recipient cell first, followed by the rest of the plasmid. Thus, the transfer of genetic material has a defined direction. Inside the recipient cell, the single strand is replicated, producing a circular, double-
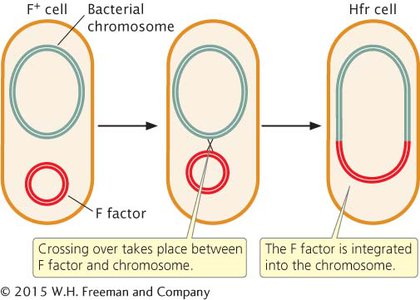
Hfr CELLS Conjugation transfers genetic material in the F plasmid from F+ to F− cells, but it does not account for the transfer of chromosomal genes observed by Lederberg and Tatum. In Hfr (high-
In conjugation between Hfr and F− cells (Figure 7.13a), the integrated F factor is nicked, and the end of the nicked strand moves into the F− cell (Figure 7.13b), just as it does in conjugation between F+ and F− cells. But because, in an Hfr cell, the F factor has been integrated into the bacterial chromosome, the chromosome follows the F factor into the recipient cell. How much of the bacterial chromosome is transferred depends on the length of time that the two cells remain in conjugation.
Inside the recipient cell, the donor DNA strand is replicated (Figure 7.13c), and crossing over between it and the original chromosome of the F− cell (Figure 7.13d) may take place. This chromosomal gene transfer between Hfr and F− cells explains how the recombinant prototrophic cells observed by Lederberg and Tatum were produced. After crossing over has taken place in the recipient cell, the donated chromosome is degraded, and the recombinant recipient chromosome remains (Figure 7.13e), to be replicated and passed on to later generations of bacterial cells by binary fission (cell division).
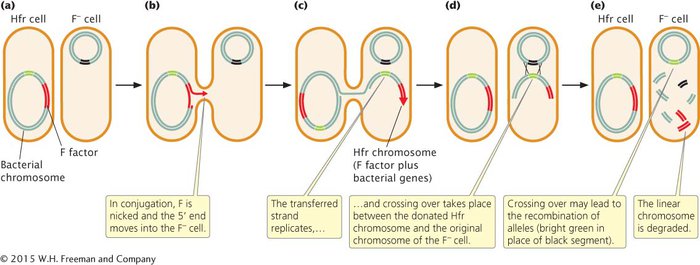
In a mating of Hfr × F−, the F− cell almost never becomes F+ or Hfr because the F factor is nicked in the middle in the initiation of strand transfer, which places part of the F factor at the beginning and part at the end of the strand that is transferred. To become F+ or Hfr, the recipient cell must receive the entire F factor, which requires that the entire donor chromosome be transferred. This event happens rarely because most conjugating cells break apart before the entire chromosome has been transferred.
The F plasmid in F+ cells integrates into the bacterial chromosome, causing an F+ cell to become Hfr, at a frequency of only about 1 in 10,000. This low frequency accounts for the low rate of recombination observed by Lederberg and Tatum in their F+ cells. The F factor is excised from the bacterial chromosome at a similarly low rate, causing a few Hfr cells to become F+.
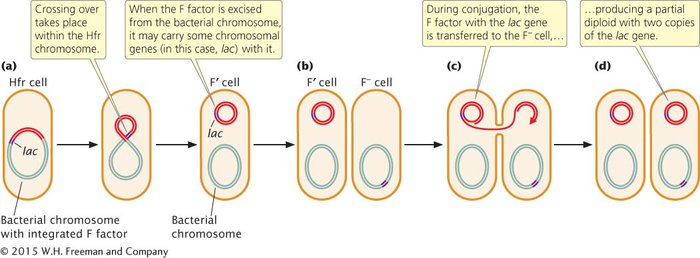
F′ CELLS When an F factor is excised from the bacterial chromosome, a small amount of the bacterial chromosome may be removed with it, and these chromosomal genes will then be carried with the F plasmid (Figure 7.14). Cells containing an F plasmid with some bacterial genes are called F prime (F′) cells. For example, if an F factor integrates into a chromosome adjacent to the lac genes (genes that enable a cell to metabolize the sugar lactose), the F factor may pick up lac genes when it is excised, becoming an F′ lac cell. F′ cells can conjugate with F− cells because F′ cells possess the F plasmid with all the genetic information necessary for conjugation and gene transfer. Characteristics of E. coli cells of different F types (called mating types) are summarized in Table 7.2.
| Type | F factor characteristics | Role in conjugation |
|---|---|---|
| F+ | Present as separate circular plasmid | Donor |
| F− | Absent | Recipient |
| Hfr | Present, integrated into bacterial chromosome | High- |
| F′ | Present as separate circular plasmid, carrying some bacterial genes | Donor |
During conjugation between an F′ cell and an F−cell, the F plasmid is transferred to the F−
| Conjugating | Cell types present after conjugation |
|---|---|
| F+ × F− | Two F+ cells (F− cell becomes F+) |
| Hfr × F− | One Hfr cell and one F− (no change)* |
| F′ × F− | Two F′ cells (F− cell becomes F′) |
|
* Rarely, the F− cell becomes F+ in an Hfr × F− conjugation if the entire chromosome is transferred during conjugation. |
|
CONCEPTS
Conjugation in E. coli is controlled by an episome called the F factor. Cells containing the F factor (F+ cells) are donors of genes; cells lacking the F factor (F− cells) are recipients. In Hfr cells, the F factor is integrated into the bacterial chromosome; these cells donate DNA to F− cells. F′ cells contain a copy of the F factor with some chromosomal genes.
 CONCEPT CHECK 3
CONCEPT CHECK 3
Conjugation between an F+ and an F− cell usually results in
two F+ cells.
two F− cells.
an F+ and an F− cell.
an Hfr cell and an F+ cell.
a
MAPPING BACTERIAL GENES WITH INTERRUPTED CONJUGATION The transfer of DNA that takes place during conjugation between Hfr and F− cells allows bacterial genes to be mapped. In conjugation, as we have seen, the chromosome of the Hfr cell is transferred to the F− cell. Transfer of the entire E. coli chromosome requires about 100 minutes; if conjugation is interrupted before 100 minutes have elapsed, only part of the donor chromosome will pass into the F− cell and have an opportunity to recombine with the recipient chromosome. Chromosome transfer always begins within the integrated F factor and proceeds in a continuous direction, so genes are transferred according to their sequence on the chromosome. This knowledge can be used to map bacterial genes by mixing Hfr and F− cells that differ in genotype and interrupting conjugation at regular intervals. The times required for individual genes to be transferred indicate their relative positions on the chromosome. In most genetic maps, distances are expressed as recombination frequencies; however, in bacterial gene maps constructed with interrupted conjugation, the basic unit of distance is a minute. View Animation 7.1 to see how genes are mapped using interrupted conjugation.
CONCEPTS
Conjugation can be used to map bacterial genes by mixing Hfr and F− cells that differ in genotype and interrupting conjugation at regular intervals. The amounts of time required for individual genes to be transferred from the Hfr to the F− cells indicate the relative positions of the genes on the bacterial chromosome.
WORKED PROBLEM
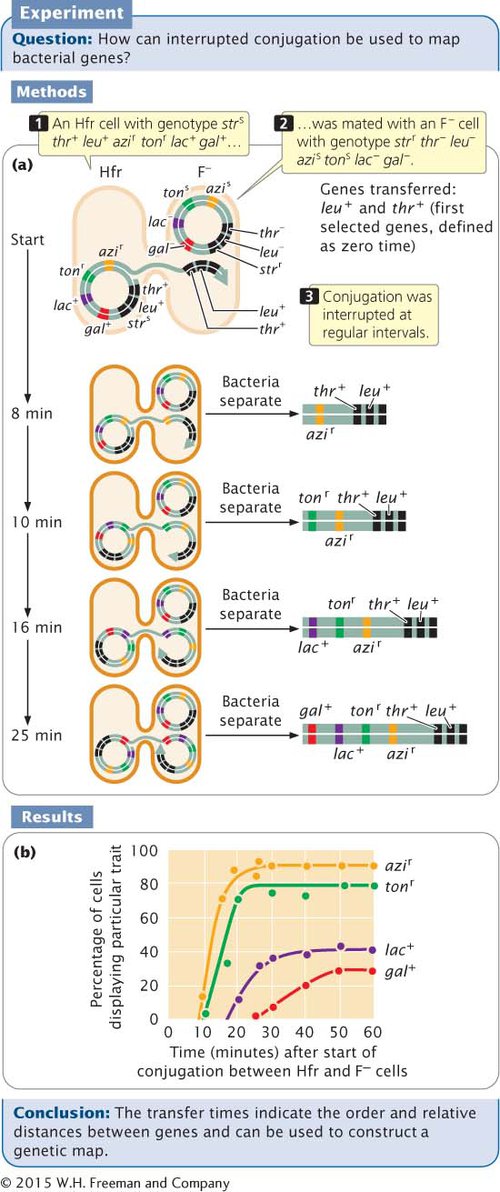
To illustrate the method of mapping genes with interrupted conjugation, let’s look at a cross analyzed by François Jacob and Elie Wollman, who developed this method of gene mapping (Figure 7.15a). They used donor Hfr cells that were sensitive to the antibiotic streptomycin (genotype strs), resistant to sodium azide (azir) and infection by bacteriophage T1 (tonr), prototrophic for threonine (thr+) and leucine (leu+), and able to break down lactose (lac+) and galactose (gal+). They used F− recipient cells that were resistant to streptomycin (strr), sensitive to sodium azide (azis) and to infection by bacteriophage T1 (tons), auxotrophic for threonine (thr−) and leucine (leu−)
Donor Hfr cells: strs leu+ thr+ azir tonr lac+ gal+
Recipient F− cells: strr leu− thr− azis tons lac− gal−
The two strains were mixed in nutrient medium and allowed to conjugate. After a few minutes, the medium was diluted to prevent any new pairings. At regular intervals, a sample of cells was removed and agitated vigorously in a kitchen blender to halt all conjugation and DNA transfer. The cells from each sample were plated on a selective medium that contained streptomycin and lacked leucine and threonine. The original donor cells were streptomycin sensitive (strs) and would not grow on this medium. The F− recipient cells were auxotrophic for leucine and threonine, and they also failed to grow on this medium. Only recipient cells that underwent conjugation and received at least the leu+ and thr+ genes from the Hfr donors could grow on this medium. All strr leu+ thr+ cells were then tested for the presence of other genes that might have been transferred from the donor Hfr strain.
Because Jacob and Wollman used streptomycin to kill all the donor cells, they were not able to examine the transfer of the strs gene. All of the cells that grew on the selective medium were leu+ thr+, so we know that these genes were transferred. In Figure 7.15b, the percentages of strr leu+ thr+ cells receiving specific alleles (azir, tonr, leu+, and gal+) from the Hfr strain are plotted against the duration of conjugation. What is the order in which the genes are transferred and what are the distances among them?
Solution Strategy
What information is required in your answer to the problem?
The order of the genes on the bacterial chromosome and the distances among them.
What information is provided to solve the problem?
The donor cells were strs leu+ thr+ azir tonr lac+ gal+ and the recipient cells were strr leu− thr− azis tons lac− gal−.
The percentages of recipient cells with different traits that appear at various times after the start of conjugation (Figure 7.15b).
Solution Steps
The first donor gene to appear in the recipient cells (at about 9 minutes) was azir. Gene tonr appeared next (after about 10 minutes), followed by lac+ (at about 18 minutes) and by gal+ (after 25 minutes) (see Figure 7.15b). These transfer times indicate the order of gene transfer and the relative distances among the genes.
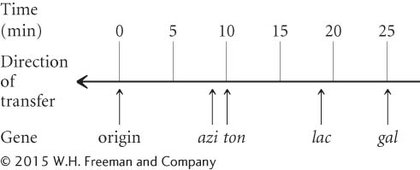
Notice that the frequency of gene transfer from donor to recipient cells decreased with distance from the origin of transfer. For example, about 90% of the recipients received the azir allele, but only about 30% received the gal+ allele. The lower percentage for gal+ is due to the fact that some conjugating cells spontaneously broke apart before they were disrupted by the blender. The probability of spontaneous disruption increases with time, so fewer cells had an opportunity to receive genes that were transferred later.
 For additional practice mapping bacterial genes with interrupted conjugation, try Problem 15 at the end of the chapter.
For additional practice mapping bacterial genes with interrupted conjugation, try Problem 15 at the end of the chapter.