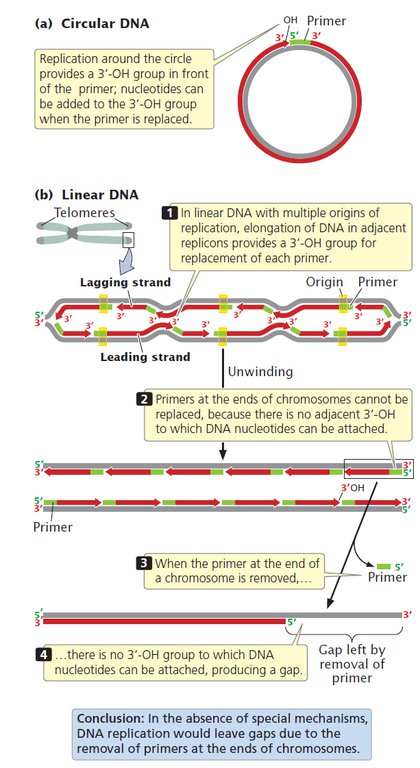
Replication at the Ends of Chromosomes
A fundamental difference between eukaryotic and bacterial replication arises because eukaryotic chromosomes are linear and thus have ends. The 3′-OH group needed for replication by DNA polymerases is provided at the initiation of replication by RNA primers that are synthesized by primase, as stated earlier. This solution is temporary because, eventually, the primers must be removed and replaced by DNA nucleotides. In a circular DNA molecule, elongation around the circle eventually provides a 3′-OH group immediately in front of the primer (Figure 9.14a). After the primer has been removed, the replacement DNA nucleotides can be added to this 3′-OH group. But what happens when a DNA molecule is not circular, but linear?
THE END-
TELOMERES AND TELOMERASE The ends of eukaryotic chromosomes—
| toward | ← | 5′—TTGGGGTTGGGG—3′ | → | end of |
| centromere | 3′—AACCCC—5′ | chromosome |
The single-
In this way, the telomerase can extend the 3′ end of the chromosome without the use of a complementary DNA template (Figure 9.15e). How the complementary C-
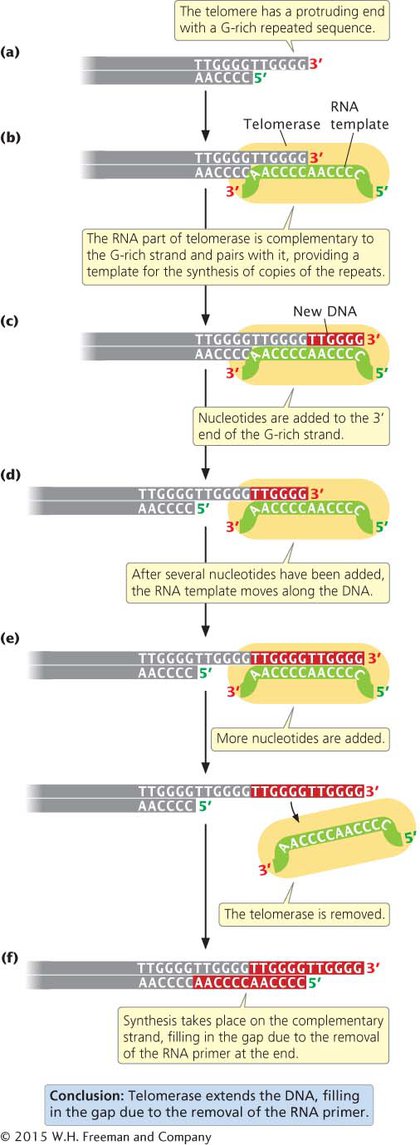
Telomerase is present in single-
CONCEPTS
The ends of eukaryotic chromosomes are replicated by an RNA–protein enzyme called telomerase. This enzyme adds extra nucleotides to the G-
 CONCEPT CHECK 7
CONCEPT CHECK 7
What would be the result if an organism’s telomerase were mutated and nonfunctional?
No DNA replication would take place.
The DNA polymerase enzyme would stall at the telomere.
Chromosomes would shorten each generation.
RNA primers could not be removed.
c
TELOMERASE, AGING, AND DISEASE The shortening of telomeres may contribute to the process of aging. The telomeres of genetically engineered mice that lack a functional telomerase gene (and therefore do not express telomerase in somatic or germ cells) undergo progressive shortening in successive generations. After several generations, these mice show some signs of premature aging, such as graying, hair loss, and delayed wound healing. Through genetic engineering, it is also possible to create somatic cells that express telomerase. In these cells, telomeres do not shorten, cell aging is inhibited, and the cells will divide indefinitely.
Some of the strongest evidence that telomere length is related to aging comes from studies of telomeres in birds. In 2012, scientists in the United Kingdom measured telomere length in red blood cells taken from 99 zebra finches at various times during their lives. The scientists found a strong correlation between telomere length and longevity; birds with longer telomeres lived longer than birds with short telomeres. The strongest predictor of life span was telomere length early in life, at 25 days, which is roughly equivalent to human adolescence. Although these observations suggest that telomere length is associated with aging in some animals, the precise role of telomeres in human aging remains uncertain.
Some diseases are associated with abnormalities of telomere replication. People with Werner syndrome, an autosomal recessive disease, show signs of premature aging that begins in adolescence or early adulthood, including wrinkled skin, graying of the hair, baldness, cataracts, and muscle atrophy. They often develop cancer, osteoporosis, heart and artery disease, and other ailments typically associated with aging. The causative gene, called WRN, has been mapped to human chromosome 8 and normally encodes a RecQ helicase enzyme, which is necessary for the efficient replication of telomeres. In people with Werner syndrome, this enzyme is defective, and consequently, the telomeres shorten prematurely.
Telomerase also appears to play a role in cancer. Cancer tumor cells have the capacity to divide indefinitely, and telomerase is expressed in 90% of all cancers. As we will see in Chapter 16, cancer is a complex, multistep process that usually requires mutations in at least several genes. Telomerase activation alone does not lead to cancerous growth in most cells, but it does appear to be required, along with other mutations, for cancer to develop. Some experimental anticancer drugs work by inhibiting the action of telomerase.
One of the difficulties in studying the effect of telomere shortening on the aging process is that the expression of telomerase in somatic cells also promotes cancer. To circumvent this problem, Antonia Tomas- TRY PROBLEM 28
TRY PROBLEM 28