CONCEPT2.4 Lipids Are Hydrophobic Molecules
Lipids—commonly called fats and oils—are hydrocarbons (composed of C and H atoms) that are insoluble in water because of their many nonpolar covalent bonds. As you have seen, nonpolar molecules are hydrophobic and preferentially aggregate together, away from polar water (see Figure 2.5). When nonpolar hydrocarbons are sufficiently close together, weak but additive van der Waals interactions (see Table 2.1) hold them together. The huge macromolecular aggregations that can form are not polymers in a strict chemical sense, because the individual lipid molecules are not covalently bonded.
Lipids play several roles in living organisms, including the following:
- They store energy in the C—C and C—H bonds.
- They play important structural roles in cell membranes and on body surfaces, largely because their nonpolar nature makes them essentially insoluble in water.
- Fat in animal bodies serves as thermal insulation.
Fats and oils are triglycerides
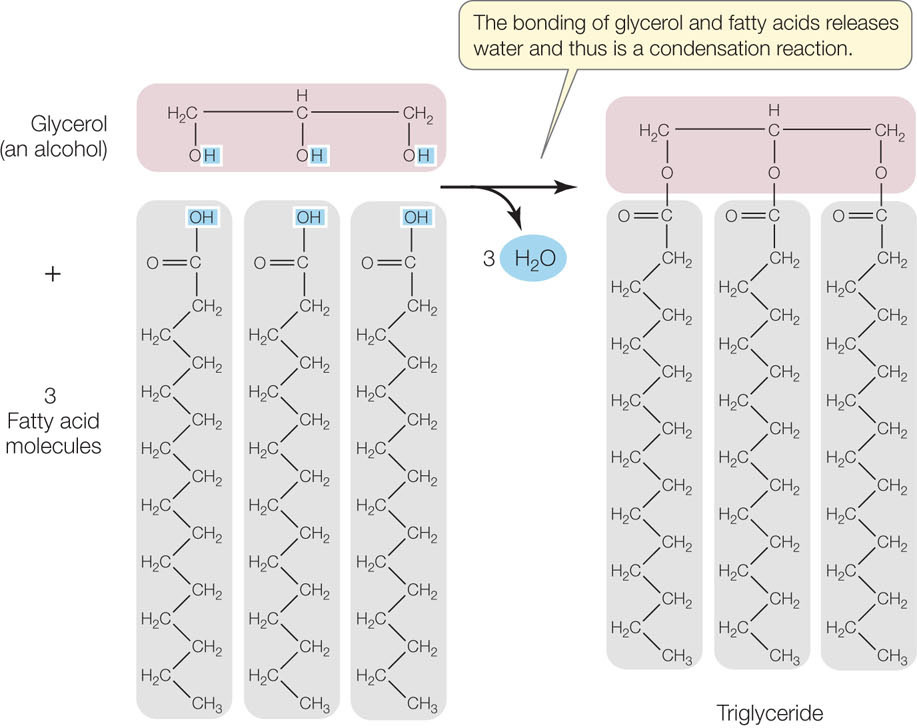
The most common units of lipids are triglycerides, also known as simple lipids. Triglycerides that are solid at room temperature (around 20°C) are called fats; those that are liquid at room temperature are called oils. A triglyceride contains three fatty acid molecules and one glycerol molecule. Glycerol is a small molecule with three hydroxyl (—OH) groups; thus it is an alcohol. A fatty acid consists of a long nonpolar hydrocarbon chain attached to the polar carboxyl (—COOH) group, and it is therefore a carboxylic acid. The long hydrocarbon chain is very hydrophobic because of its abundant C—H and C—C bonds.
Synthesis of a triglyceride involves three condensation reactions (FIGURE 2.11). The resulting molecule has very little polarity and is extremely hydrophobic. That is why fats and oils do not mix with water but float on top of it in separate globules or layers. The three fatty acids in a single triglyceride molecule need not all have the same hydrocarbon chain length or structure; some may be saturated fatty acids, while others may be unsaturated:
- In a saturated fatty acid, all the bonds between the carbon atoms in the hydrocarbon chain are single; there are no double bonds. That is, all the available bonds are saturated with hydrogen atoms (FIGURE 2.12A). These fatty acid molecules are relatively rigid and straight, and they pack together tightly, like pencils in a box.
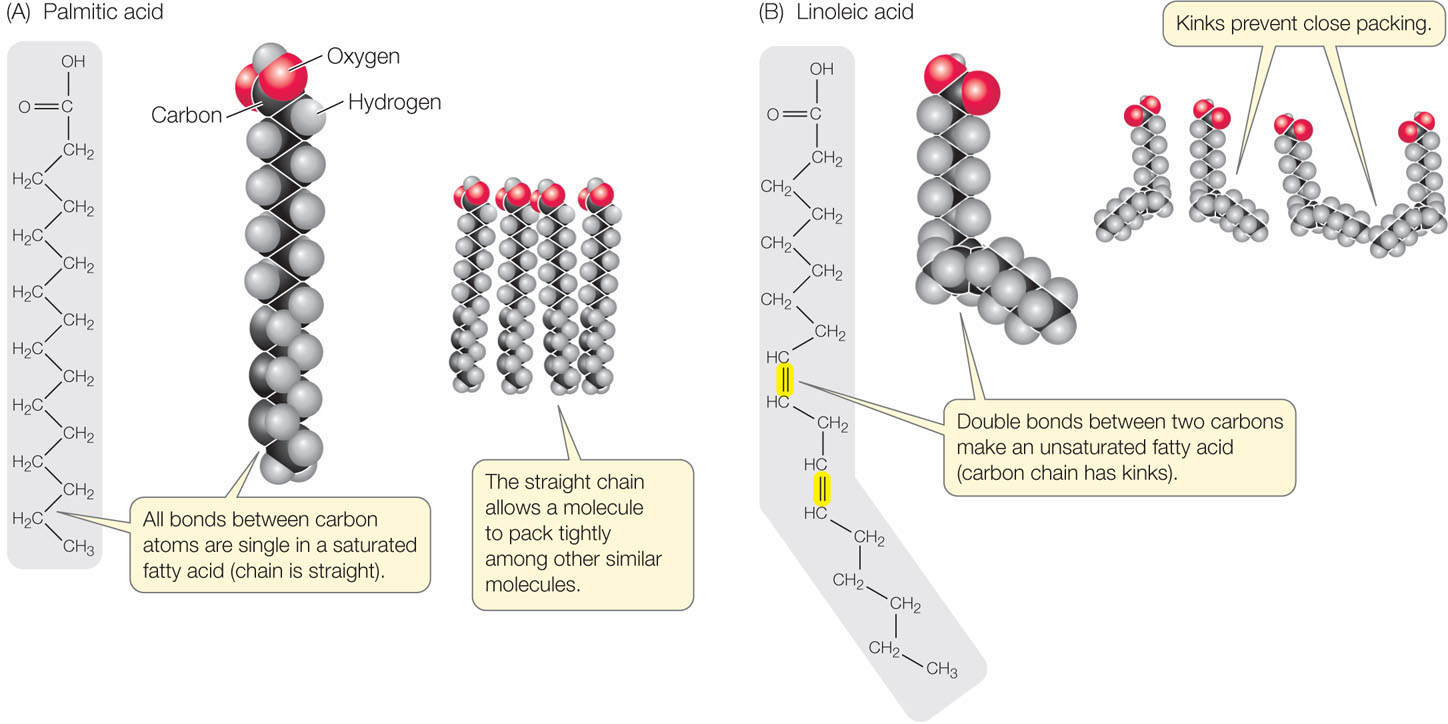 Figure 2.12: Saturated and Unsaturated Fatty Acids (A) The straight hydrocarbon chain of a saturated fatty acid allows the molecule to pack tightly with other, similar molecules. (B) In unsaturated fatty acids, kinks in the chain prevent close packing.
Figure 2.12: Saturated and Unsaturated Fatty Acids (A) The straight hydrocarbon chain of a saturated fatty acid allows the molecule to pack tightly with other, similar molecules. (B) In unsaturated fatty acids, kinks in the chain prevent close packing. - In an unsaturated fatty acid, the hydrocarbon chain contains one or more double bonds. Linoleic acid is an example of a polyunsaturated fatty acid that has two double bonds near the middle of the hydrocarbon chain, causing kinks in the chain (FIGURE 2.12B). Such kinks prevent the unsaturated molecules from packing together tightly.
The kinks in fatty acid molecules are important in determining the fluidity and melting point of the lipid. The triglycerides of animal fats tend to have many long-chain saturated fatty acids, which pack tightly together; these fats are usually solid at room temperature and have a high melting point. The triglycerides of plants, such as corn oil, tend to have short or unsaturated fatty acids. Because of their kinks, these fatty acids pack together poorly, have low melting points, and are usually liquid at room temperature.
30

Fats and oils are excellent storehouses for chemical energy. As you will see in Chapter 6, energy is released when these compounds are broken down into smaller molecules. The released energy can be used by an organism for other purposes, such as movement or the synthesis of complex molecules. On a per weight basis, broken-down lipids yield more than twice as much energy as degraded carbohydrates.
Phospholipids form biological membranes
We have mentioned the hydrophobic nature of the many C—C and C—H bonds in a fatty acid. But what about the carboxyl functional group at the end of the molecule? When it ionizes and forms COO−, it is strongly hydrophilic. So a fatty acid has two opposing chemical properties: a hydrophilic end and a long hydrophobic tail. A molecule that is partly hydrophilic and partly hydrophobic is said to be amphipathic.
In triglycerides, a glycerol molecule is bonded to three fatty acid chains and the resulting molecule is entirely hydrophobic. Phospholipids are like triglycerides in that they contain fatty acids bound to glycerol. However, in phospholipids, a phosphate-containing compound replaces one of the fatty acids, giving these molecules amphipathic properties (FIGURE 2.13A). The phosphate functional group (there are several different kinds in different phospholipids) has a negative electric charge, so this portion of the molecule is hydrophilic, attracting polar water molecules. But the two fatty acids are hydrophobic, so they tend to avoid water and aggregate together or with other hydrophobic substances.
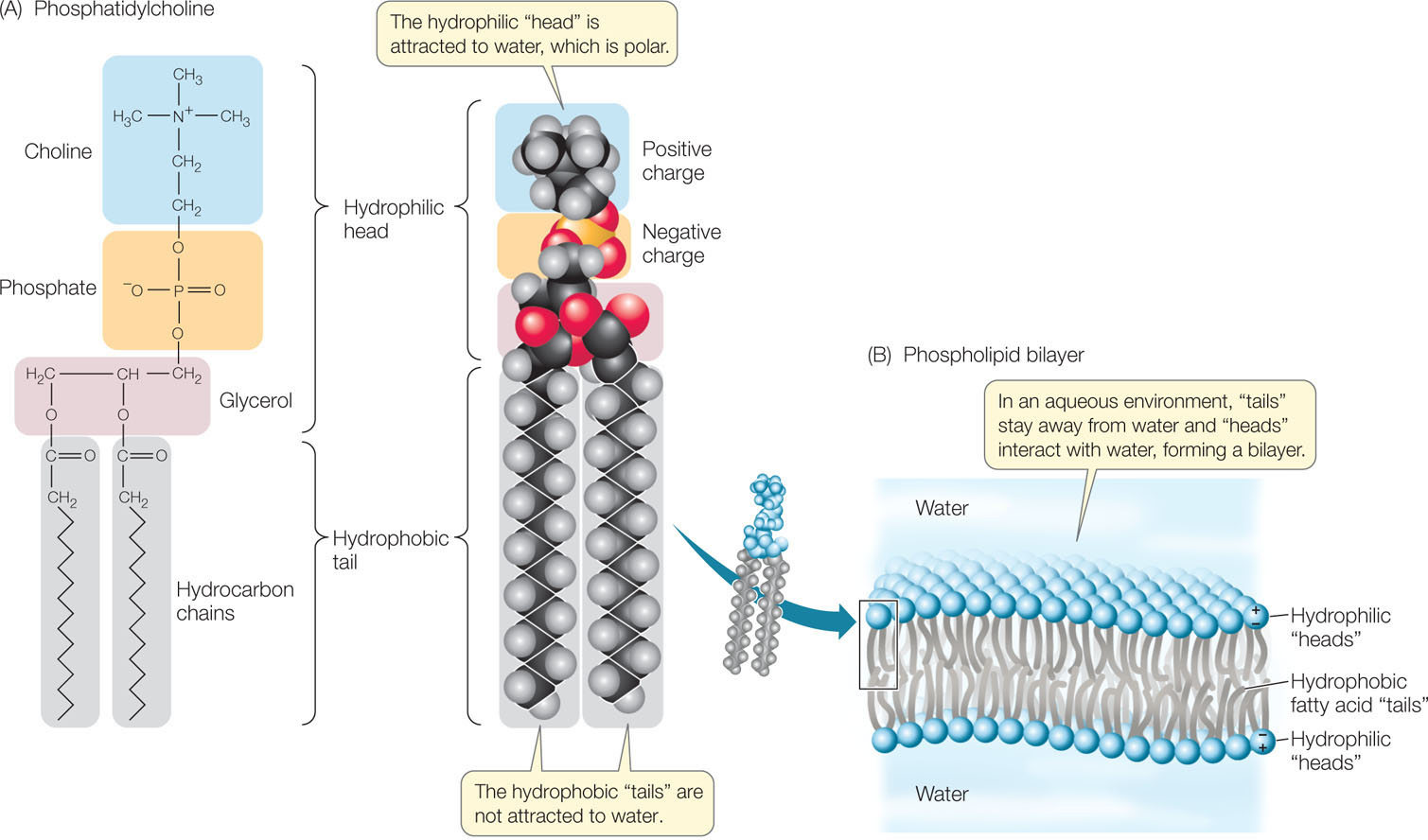
In an aqueous environment, phospholipids line up in such a way that the nonpolar, hydrophobic “tails” pack tightly together and the phosphate-containing “heads” face outward, where they interact with water. The phospholipids thus form a bilayer: a sheet two molecules thick, with water excluded from the core (FIGURE 2.13B). Although no covalent bonds link individual lipids in these large aggregations, such stable aggregations form readily in aqueous conditions. Biological membranes have this kind of phospholipid bilayer structure, and we will devote Chapter 5 to their biological functions.
CHECKpointCONCEPT2.4
- What is the difference between fats and oils?
- Why are phospholipids amphipathic, and how does this result in a lipid bilayer membrane?
- If fatty acids are carefully put onto the surface of water, they form a single molecular layer. If the mixture is then shaken vigorously, the fatty acids will form round structures called micelles. Explain these observations.
31
Molecules such as carbohydrates and lipids are not always stable in living systems. Rather, a hallmark of life is its ability to transform molecules. This involves making and breaking covalent bonds, as some atoms are removed and others are attached. As part of our introduction to biochemical concepts, we will now turn to these processes of chemical change.