CONCEPT5.2 Passive Transport across Membranes Requires No Input of Energy
An important property of all life is the ability to regulate the internal composition of a cell, distinguishing it from the surrounding environment. Biological membranes allow some substances, but not others, to pass through them. This characteristic of membranes is called selective permeability. If a membrane is permeable to a particular substance, that substance can simply diffuse (as we describe below) across the membrane from a region of higher concentration to a region of lower concentration. However, some substances must be transported across membranes, and this process is facilitated by specialized membrane proteins.
There are two fundamentally different processes by which substances cross biological membranes:
- The processes of passive transport do not require direct inputs of metabolic energy to drive them. In general, passive transport occurs when a substance moves from the side of the membrane where its concentration is higher to the side where its concentration is lower. In other words, the substance moves down its concentration gradient. Passive transport can also occur when an ion is transported across a membrane to reduce charge differences between the two sides of the membrane.
- The processes of active transport require the input of metabolic energy because they involve the movement of substances against their concentration gradients. Even if the membrane is permeable to a substance, that substance must be actively transported from the side where its concentration is lower to the side where it is higher.
This section focuses on passive transport across the membrane. Passive transport can occur by simple diffusion through the phospholipid bilayer, or it can be facilitated by channel proteins or carrier proteins.
Simple diffusion takes place through the phospholipid bilayer
In a solution, there is a tendency for all of the components to be evenly distributed. You can see this when a drop of ink is allowed to fall into a gelatin suspension (a “gel”). Initially the pigment molecules are very concentrated, but they will move about at random, slowly spreading until the intensity of color is exactly the same throughout the gel:
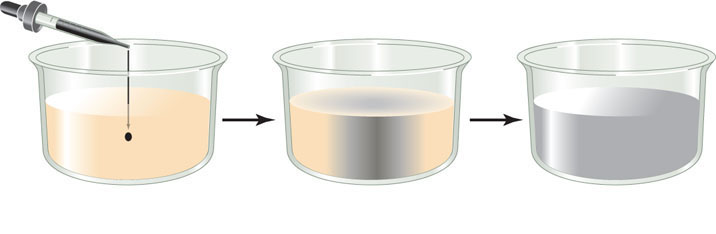
A solution in which the solute molecules are uniformly distributed is said to be at equilibrium. This does not mean the molecules have stopped moving; it just means they are moving in such a way that their overall distribution does not change.
Diffusion is the process of random movement toward a state of equilibrium. In effect, it is a net movement from regions of greater concentration to regions of lesser concentration. Diffusion is generally a very slow process in living tissues except over short distances, especially when we consider the gel-like consistency of the cell cytoplasm. For example, it would take about 3 years for a molecule of oxygen gas (O2) to diffuse from the human lung to a cell at the fingertip! So it is not surprising that as plants and animals evolved and became larger and multicellular, those with circulatory systems to distribute vital molecules such as O2 had a distinct advantage over organisms relying on simple diffusion.
How fast a substance diffuses depends on three factors:
- The diameter of the molecules or ions: smaller molecules diffuse faster.
- The temperature of the solution: higher temperatures lead to faster diffusion because the heat provides more energy for movement.
- The concentration gradient in the system—that is, the change in solute concentration with distance in a given direction. The greater the concentration gradient, the more rapidly a substance diffuses.
88
What does this mean for a cell surrounded by a membrane? The cytoplasm is largely a water-based (aqueous) solution, and so is the surrounding environment. In a complex solution (one with many different solutes), the diffusion of each solute depends only on its own concentration, not on the concentrations of other solutes. So one might expect a substance with a higher concentration inside the cell to diffuse out, and one with a higher concentration outside the cell to diffuse in. Indeed, some small molecules can pass through the phospholipid bilayer of the membrane by simple diffusion. Gases, including oxygen and carbon dioxide, can cross membranes this way. Small nonpolar and uncharged molecules can enter the membrane readily and pass through it. The more lipid-soluble the molecule is, the more rapidly it diffuses through the lipid bilayer.
In contrast, electrically charged or polar (hydrophilic) molecules, such as amino acids, sugars, ions, and water, do not pass readily through a membrane because they are not soluble in the hydrophobic interior of the lipid bilayer. However, as we discuss below, specialized proteins facilitate the transport of these molecules across membranes.
Osmosis is the diffusion of water across membranes
Water molecules pass through specialized channels in membranes (see below) by a diffusion process called osmosis. This process depends on the relative concentrations of water molecules on both sides of the membrane. In a particular solution, the higher the total solute concentration, the lower the concentration of water molecules. Osmotic pressure is defined as the pressure that needs to be applied to a solution to prevent the flow of water across a membrane by osmosis. This pressure is proportional to the total concentration of solutes in the solution—the more dissolved solutes there are, the fewer water molecules there are, and so water moves across the membrane and into the solution. The equation for osmotic pressure (symbolized by the Greek capital letter pi, Π) due to water is
Π = cRT
where c is total solute concentration, R is the gas constant, and T is the absolute temperature. In thermodynamic terms, the higher concentration of a substance in a compartment on one side of a membrane represents stored energy.
Consider a situation where a membrane separating two different solutions allows water, but not solutes, to pass through. The water molecules will move across the membrane toward the solution with the higher solute concentration and the lower concentration of water molecules.
Here we are referring to the net movement of water. Since it is so abundant, water is constantly moving (through channel proteins) across the cell membrane, into and out of cells. But if there is a concentration difference between the two sides of the membrane, the overall movement will be greater in one direction or the other.
Three terms are used to compare the solute concentrations of two solutions separated by a membrane:
- A hypertonic solution has a higher solute concentration than the other solution (FIGURE 5.3A).
- Isotonic solutions have equal solute concentrations (FIGURE 5.3B).
- A hypotonic solution has a lower solute concentration than the other solution (FIGURE 5.3C).
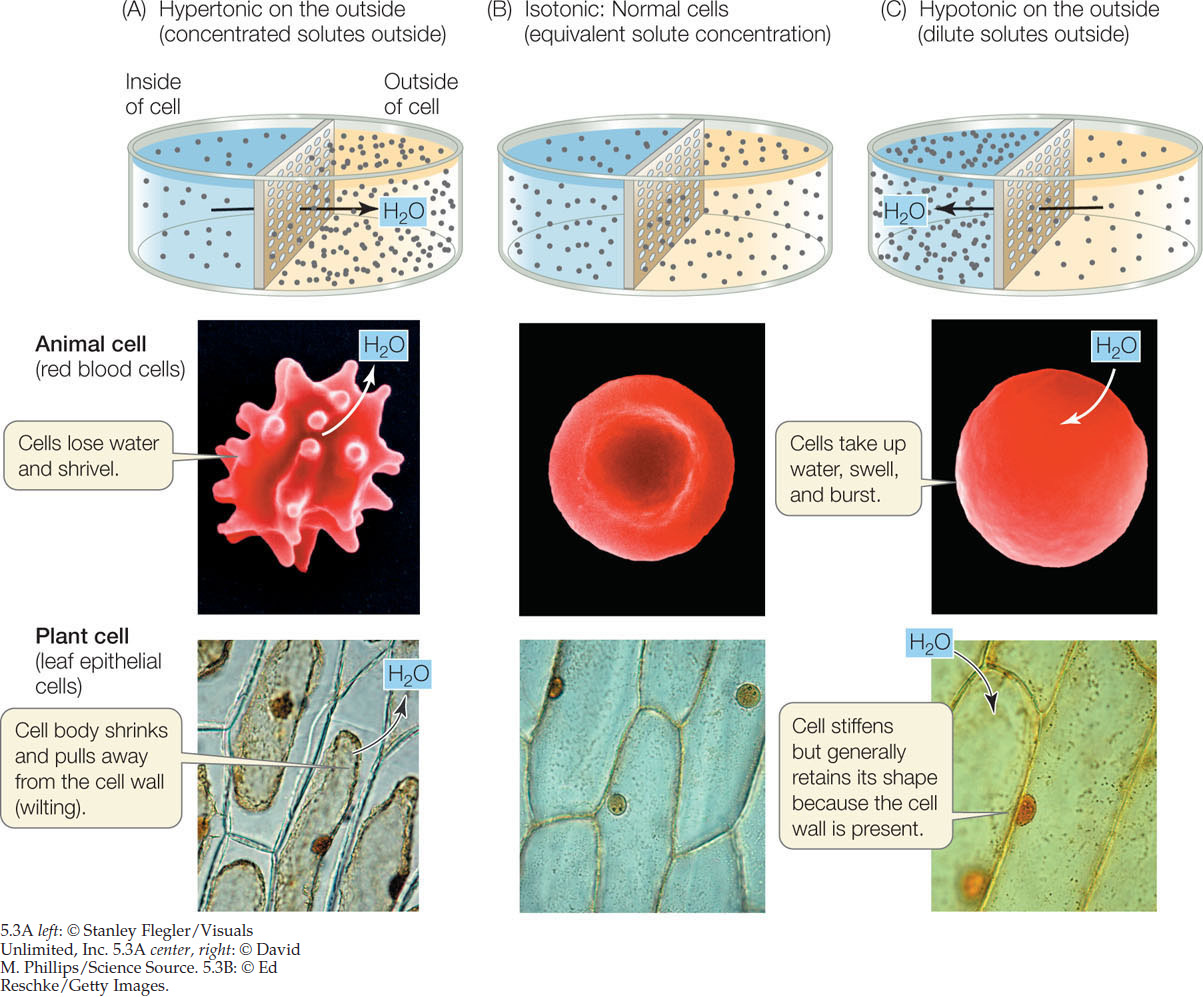
The concentration of solutes in the environment determines the direction of osmosis in all animal cells. A red blood cell takes up water from a solution that is hypotonic to the cell’s contents. If this happens, the cell bursts because its cell membrane cannot withstand the pressure created by the water entry and the resultant swelling (see Figure 5.3C). Conversely, the cell shrinks if the solution surrounding it is hypertonic to its contents (see Figure 5.3A). The integrity of blood cells is absolutely dependent on the maintenance of a constant solute concentration in the surrounding blood plasma—the plasma must be isotonic to the blood cells. Regulation of the solute concentrations of body fluids is thus an important process for organisms without cell walls.
In contrast to animal cells, the cells of plants, archaea, bacteria, fungi, and some protists have cell walls that limit their volumes and keep them from bursting. Cells with sturdy walls take up a limited amount of water, and in so doing they build up internal pressure against the cell wall, which prevents further water from entering. This pressure within the cell is called turgor pressure; it keeps the green parts of plants upright and is the driving force for enlargement of plant cells (see Concept 25.3). It is a normal and essential component of plant growth. If enough water leaves the cells, turgor pressure drops and the plant wilts. Turgor pressure reaches about 100 pounds per square inch (0.7 kg/cm2), which is greater than the pressure in auto tires (about 35 pounds per square inch).
LINK
The roles of osmosis in plant physiological processes are described in Concept 25.3. Excretion in animals also involves osmosis; see Concept 36.1
Diffusion may be aided by channel proteins
As we saw earlier, polar or charged substances such as water, amino acids, sugars, and ions do not readily diffuse across membranes. But they can cross the hydrophobic phospholipid bilayer passively (that is, without the input of energy) in one of two ways, depending on the substance:
- Channel proteins are integral membrane proteins that form channels across the membrane through which certain substances can pass.
- Some substances can bind to membrane proteins called carrier proteins that speed up their diffusion through the phospholipid bilayer.
Both of these processes are forms of facilitated diffusion. The substances diffuse according to their concentration gradients, but their diffusion is made easier by channel or carrier proteins. Particular channel or carrier proteins allow diffusion both into and out of a cell or organelle. In other words, they can operate in both directions.
We will focus here on two examples of channel proteins and discuss carrier proteins in the next section.
Ion Channels
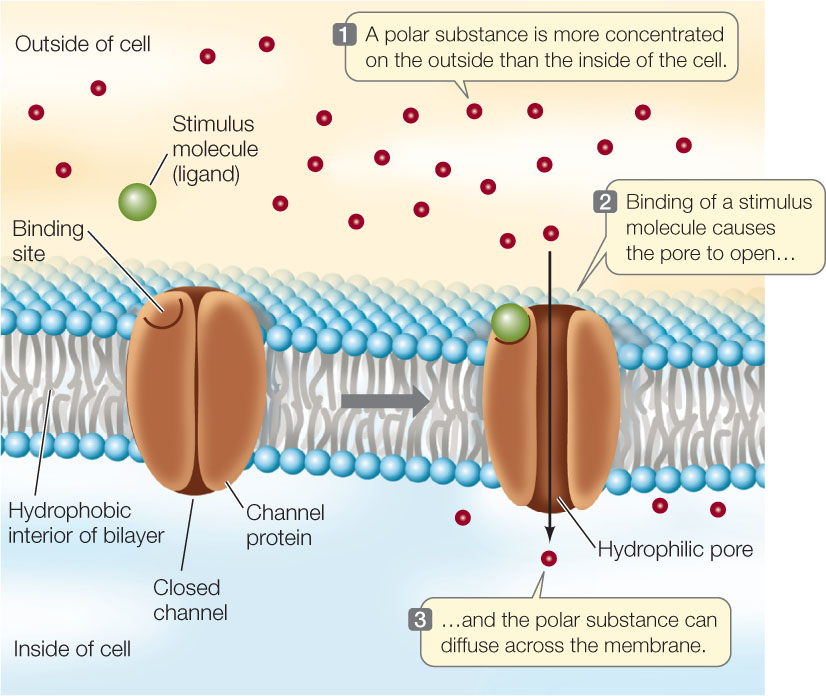
The best-studied channel proteins are the ion channels. As you will see in later chapters, the movement of ions across membranes is important in many biological processes, including ATP production within the mitochondria, the electrical activity of the nervous system, and the opening of pores in plant leaves to allow gas exchange with the environment. Several types of ion channels have been identified, each of them specific for a particular ion. All of them show the same basic structure of a hydrophilic pore that allows a particular ion to move through it.
Just as a fence may have a gate that can be opened or closed, most ion channels are “gated”: they can be opened or closed to ion passage. A gated channel opens when a stimulus causes a change in the three-dimensional shape of the channel. In some cases, this stimulus is the binding of a chemical signal, or ligand. Channels controlled in this way are called ligand-gated channels (FIGURE 5.4). In contrast, a voltage-gated channel is stimulated to open or close by a change in the voltage (electrical charge difference) across the membrane (see Figure 34.5).
Aquaporins
Water crosses membranes at a much faster rate than would be expected if it simply diffused through the phospholipid bilayer. One way water does this is by “hitchhiking” with some ions, such as Na+, as they pass through ion channels. Up to 12 water molecules may coat an ion as it traverses a channel. But there is an even faster way for water to cross membranes. Plants and some animal cells (such as red blood cells and kidney cells) have membrane channels called aquaporins. These specific channels allow large amounts of water to move down its concentration gradient, as you will see when we discuss water relations in plants (see Chapter 25) and animals (see Chapter 36).
90
Aquaporins were first identified by Peter Agre at Duke University. He noticed a membrane protein that was present in red blood cells, kidney cells, and plant cells, all of which show rapid diffusion of water across their membranes. To test the idea that the membrane protein might be a water channel, Agre injected egg cells (oocytes) with the mRNA for the protein. The injected cells produced the protein and inserted it into their membranes. An oocyte membrane does not normally permit much diffusion of water. However, the injected oocytes began swelling immediately after being transferred to a hypotonic solution, indicating the rapid diffusion of water into the cells (FIGURE 5.5).
Investigation
HYPOTHESIS
Aquaporin increases membrane permeability to water.
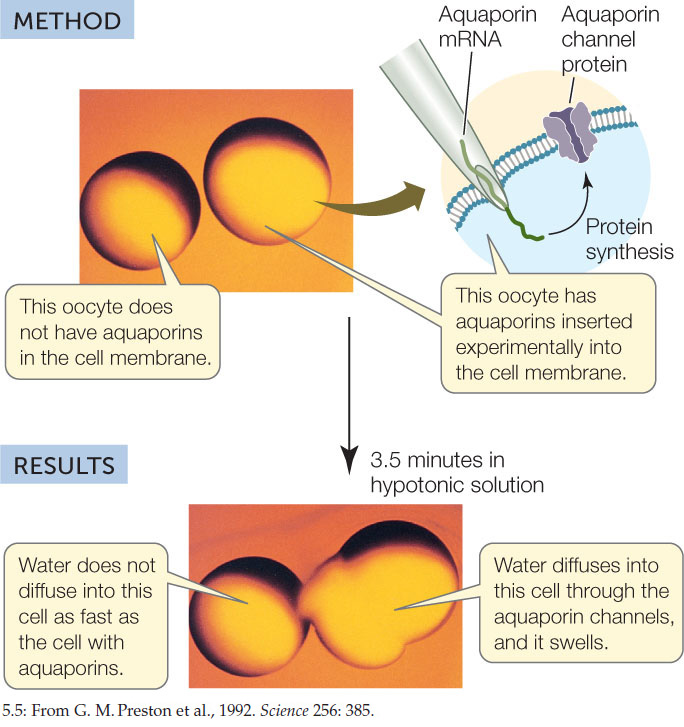
CONCLUSION
Aquaporin increases the rate of water diffusion across the cell membrane.
ANALYZE THE DATA
Oocytes were injected with aquaporin mRNA (red circles) or a solution without mRNA (blue circles). Water permeability was tested by incubating the oocytes in hypotonic solution and measuring cell volume. After time X in the upper curve, intact oocytes were not visible:
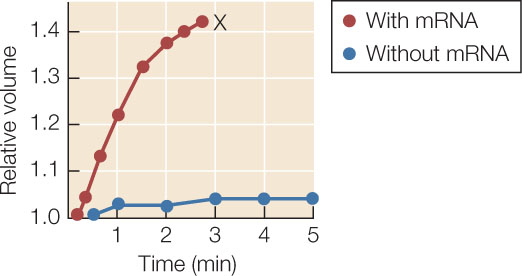
- Why did the cells with aquaporin mRNA increase in volume?
- What happened at time X?
- Calculate the relative rates (volume increase per minute) of swelling in the control and experimental curves. What does this show about the effectiveness of mRNA injection?
a G. M. Preston et al. 1992. Science 256: 385–387.
Carrier proteins aid diffusion by binding substances
Another kind of facilitated diffusion involves the actual binding of the transported substance to a membrane protein called a carrier protein. Carrier proteins transport polar molecules such as sugars and amino acids.
Glucose is the major energy source for most mammalian cells, and they require a great deal of it. Their membranes contain a carrier protein—the glucose transporter—that facilitates glucose uptake into the cell. Binding of glucose to a specific three-dimensional site on one side of the transport protein causes the protein to change its shape and release glucose on the other side of the membrane (FIGURE 5.6A). Since glucose is usually broken down as soon as it enters the cell, there is almost always a strong concentration gradient favoring glucose entry (that is, a higher concentration outside the cell than inside). The transporter allows glucose molecules to cross the membrane and enter the cell much faster than they would by simple diffusion through the bilayer. This rapid entry is necessary to ensure that the cell receives enough glucose for its energy needs.
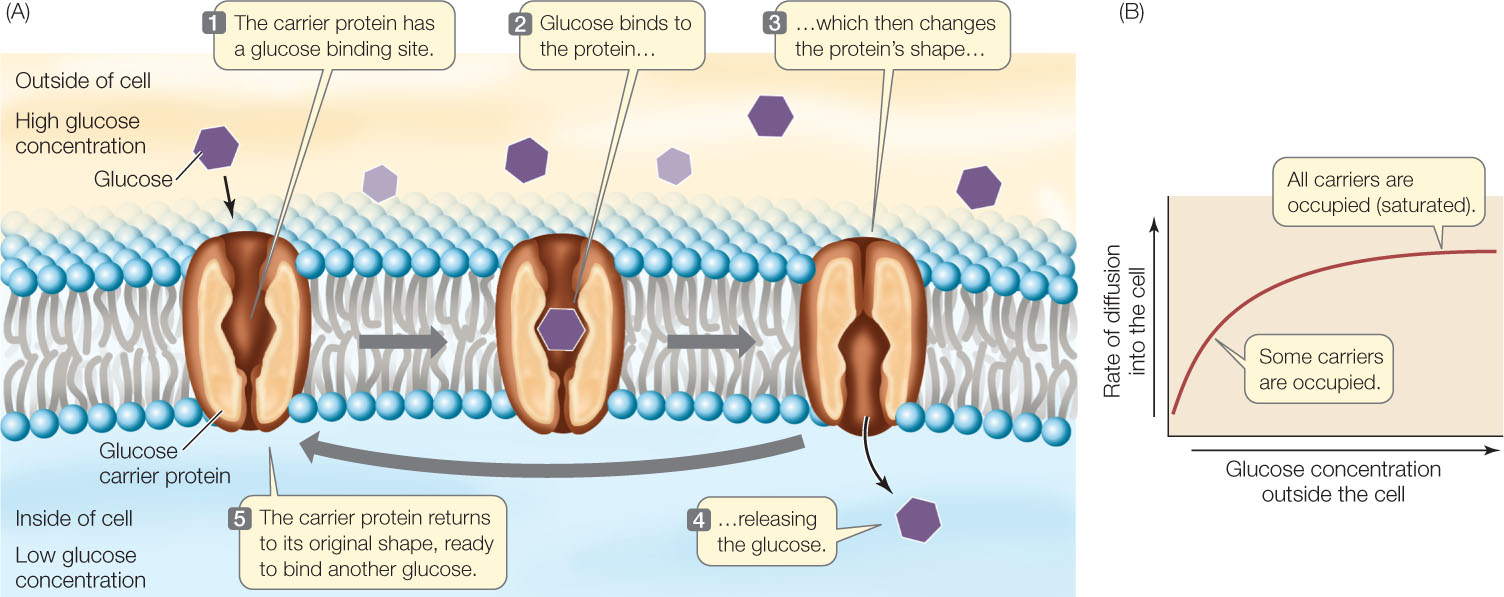
Transport by carrier proteins is different from simple diffusion. In both processes, the rate of movement depends on the concentration gradient across the membrane. However, in carrier-mediated transport, a point is reached at which increases in the concentration gradient are not accompanied by an increased rate of diffusion. At this point, the facilitated diffusion system is said to be saturated (FIGURE 5.6B). Because there are only a limited number of carrier protein molecules per unit of membrane area, the rate of diffusion reaches a maximum when all the carrier molecules are fully loaded with solute molecules. This situation is similar to that of enzyme saturation (see Figure 3.16).
91
CHECKpointCONCEPT5.2
- What properties of a substance determine whether, and how fast, it will diffuse across a membrane?
- Compare the process of facilitated diffusion through a channel and by a carrier protein. Which might be faster, and why?
- After celery is stored in an open, dry container in the refrigerator for two days, it is wilted. However, immersing the cut stalk in water for a few hours restores the integrity of the celery. How?
Diffusion tends to equalize the concentrations of substances between the outsides and insides of cells or organelles. However, one hallmark of a living thing is that it can have an internal composition quite different from that of its environment. To achieve this, a cell must sometimes move substances against their concentration gradients. This process requires work—the input of energy—and is known as active transport.