CONCEPT6.3 Carbohydrate Catabolism in the Absence of Oxygen Releases a Small Amount of Energy
In the absence of O2—that is, when conditions are anaerobic—the respiratory chain cannot operate. (The exceptions, as we noted earlier, are the respiratory chains of anaerobic microbes adapted to use terminal electron acceptors other than oxygen.) Without an alternative, the NADH produced by glycolysis would not be reoxidized and glycolysis would stop, because there would be no NAD+ for step 6 of glycolysis (see Figure 6.7). To solve this problem, organisms use fermentation to reoxidize the NADH, thus allowing glycolysis to continue (FIGURE 6.12).
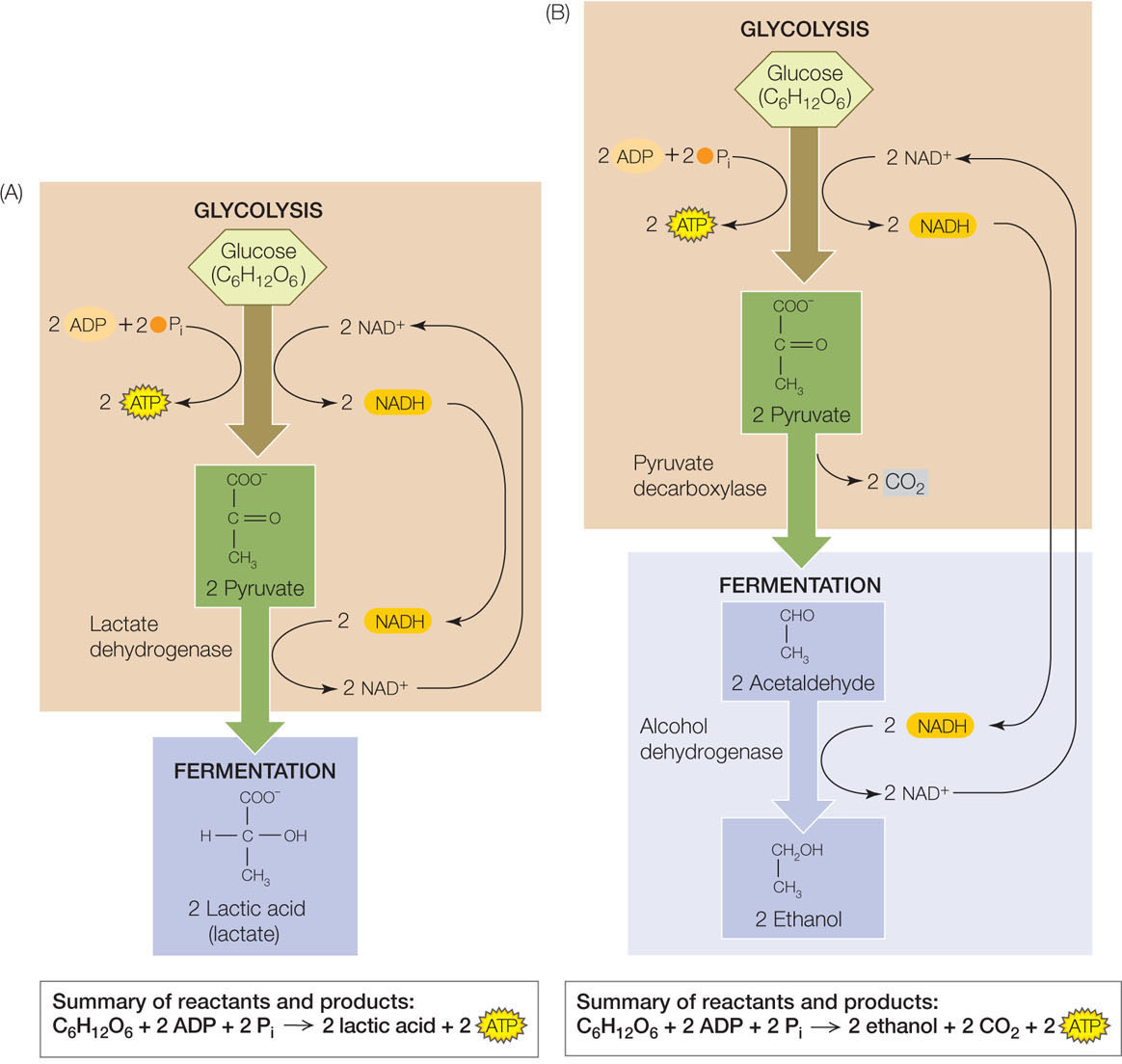
Like glycolysis, fermentation pathways occur in the cytoplasm. There are many different types of fermentation used by different organisms, but all operate to regenerate NAD+. The consequence of this is that the NADH made during glycolysis is not available for reoxidation by the respiratory chain to form ATP. Therefore the overall yield of ATP from fermentation is restricted to the ATP made in glycolysis (two ATP per glucose).
116
Two fermentation pathways are found in a wide variety of organisms:
- Lactic acid fermentation, whose end product is lactic acid (lactate)
- Alcoholic fermentation, whose end product is ethyl alcohol (ethanol)
In lactic acid fermentation, pyruvate serves as the electron acceptor and lactate is the product (see Figure 6.12A). This process takes place in many microorganisms and complex organisms, including more complex plants and vertebrates. A notable example of lactic acid fermentation occurs in vertebrate muscle tissue. Usually, vertebrates get their energy for muscle contractions aerobically, with the circulatory system supplying O2 to muscles. This is almost always adequate for small vertebrates, which explains why birds can fly long distances without resting. But in larger vertebrates such as humans, the circulatory system is not up to the task of delivering enough O2 when the need is great, such as during a long sprint. At this point, the muscle cells break down glycogen (a stored polysaccharide) and undergo lactic acid fermentation. The process is reversible; lactate is converted back to pyruvate once O2 is available again.
Alcoholic fermentation takes place in certain yeasts (eukaryotic microbes) and some plant cells under anaerobic conditions. In this process, pyruvate is converted to ethanol (see Figure 6.12B). We saw these reactions (as did Pasteur) in the opening story of this chapter. As with lactic acid fermentation, the reactions are essentially reversible.
By recycling NAD+, fermentation allows glycolysis to continue, thus producing small amounts of ATP through substrate-level phosphorylation. The net yield of two ATPs per glucose molecule is much lower than the energy yield from oxidative phosphorylation. For this reason, most organisms that rely on fermentation instead of respiration are small microbes that grow relatively slowly.
Although its yield of ATP per glucose is generally low, in some circumstances cellular anaerobic metabolism can produce an adequate supply of ATP if the enzymatic reactions in the pathway are speeded up. Indeed, this occurs in vertebrate muscle cells (see Concept 33.3) and in some cancer cells where O2 is in low supply.
CHECKpointCONCEPT6.3
- Why is replenishing NAD+ crucial to cellular metabolism?
- Compare the sources and total energy yield in terms of ATP per glucose in human cells in the presence versus the absence of O2.
- Conditions can become anaerobic in a heart muscle cell during a heart attack, because of the inadequate supply of blood. If O2 is restored, what will happen to the lactate produced by the heart muscle?
You have seen how cells harvest chemical energy in cellular respiration. Now we will see how that energy moves through other metabolic pathways in the cell.