CONCEPT10.4 Translation of the Genetic Code Is Mediated by tRNAs and Ribosomes
The translation of mRNA into proteins requires molecules that can link the information contained in each mRNA codon with a specific amino acid. That function is performed by a set of transfer RNAs (tRNAs). Two key events must take place to ensure that the protein made is the one specified by the mRNA:
- A tRNA must chemically read each mRNA codon correctly.
- The tRNA must deliver the amino acid that corresponds to the mRNA codon.
Once the tRNAs “decode” the mRNA and deliver the appropriate amino acids, components of the ribosome catalyze the formation of peptide bonds between the amino acids.
Transfer RNAs carry specific amino acids and bind to specific codons
There is at least one specific tRNA molecule for each of the 20 amino acids. Each tRNA has three functions that are fulfilled by its structure and base sequence (FIGURE 10.13):
- tRNAs bind to particular amino acids. Each tRNA binds to a specific enzyme that attaches it to only 1 of the 20 amino acids. This covalent attachment is at the 3′ end of the tRNA. We will describe the details of this vital process in the next section. When it is carrying an amino acid, the tRNA is said to be “charged.”
- tRNAs bind to mRNA. At about the midpoint on the tRNA polynucleotide chain there is a triplet of bases called the anticodon, which is complementary to the mRNA codon for the particular amino acid that the tRNA carries. Like the two strands of DNA, the codon and anticodon bind together via noncovalent hydrogen bonds. For example, the mRNA codon for arginine is 5′-CGG-3′, and the tRNA anticodon is 3′-GCC-5′.
- tRNAs interact with ribosomes. The ribosome has several sites on its surface that just fit the three-dimensional structure of a tRNA molecule. Interaction between the ribosome and the tRNA is noncovalent.
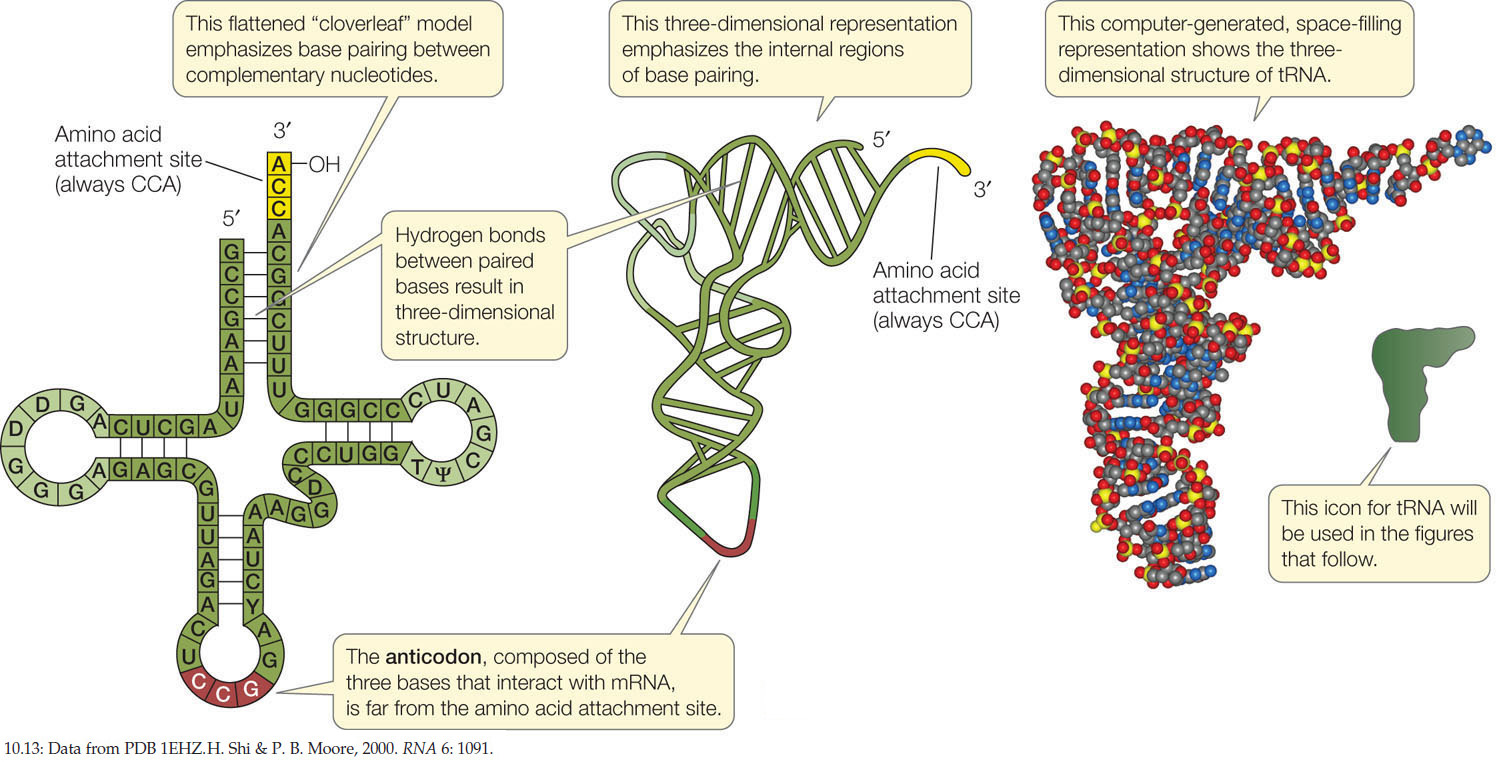
Recall that 61 different codons encode the 20 amino acids in proteins (see Figure 10.11). Does this mean that the cell must produce 61 different tRNA species, each with a different anticodon? No. The cell gets by with about two-thirds of that number of tRNA species because the specificity for the base at the 3′ end of the codon (and the 5′ end of the anticodon) is not always strictly observed. This phenomenon is called “wobble,” and it occurs because in some cases unusual or modified nucleotide bases occur in the 5′ position of the anticodon. One such unusual base is inosine (I), which can pair with A, C, and U. For example, its presence allows three of the alanine codons—GCA, GCC, and GCU—to be recognized by the same tRNA (with the anticodon 3′-CGI-5′). Wobble occurs in some matches but not in others; of most importance, it does not allow the genetic code to be ambiguous. That is, each mRNA codon binds to just one tRNA species, carrying a specific amino acid.
Each tRNA is specifically attached to an amino acid
The charging of each tRNA with its correct amino acid is achieved by a family of enzymes known as aminoacyl-tRNA synthases. Each enzyme is specific for one amino acid and for its corresponding tRNA. The reaction uses the energy in ATP to form a high-energy bond between the amino acid and the tRNA:
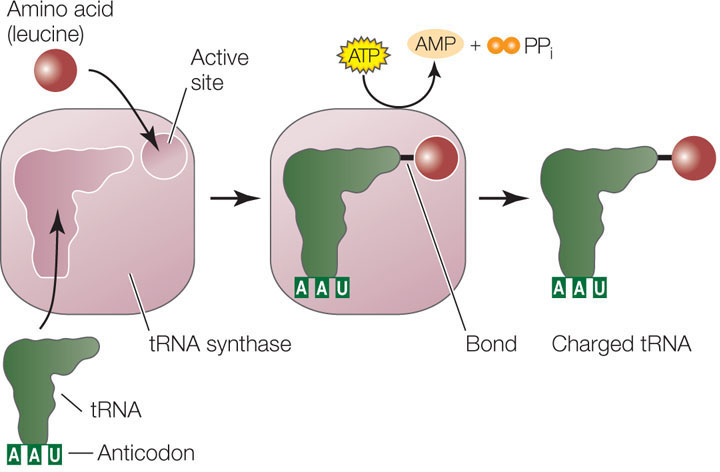
The energy in this bond is later used in the formation of peptide bonds between amino acids in a growing polypeptide chain. Clearly, the specificity between the tRNA and its corresponding amino acid is extremely important. These two reactions, for example, are highly specific:

A clever experiment by Seymour Benzer and his colleagues at Purdue University demonstrated the importance of this specificity. They took the Cys-tRNAcys molecule and chemically converted the cysteine into alanine, resulting in Ala-tRNAcys. Which component—the amino acid or the tRNA—would be recognized when this hybrid charged tRNA was put into a protein-synthesizing system? The answer was the tRNA. Everywhere in the synthesized protein where cysteine was supposed to be, alanine appeared instead. The cysteine-specific tRNA had delivered its cargo (alanine) to every mRNA codon for cysteine. This experiment showed that the protein synthesis machinery recognizes the anticodon of the charged tRNA, not the amino acid attached to it.
Translation occurs at the ribosome
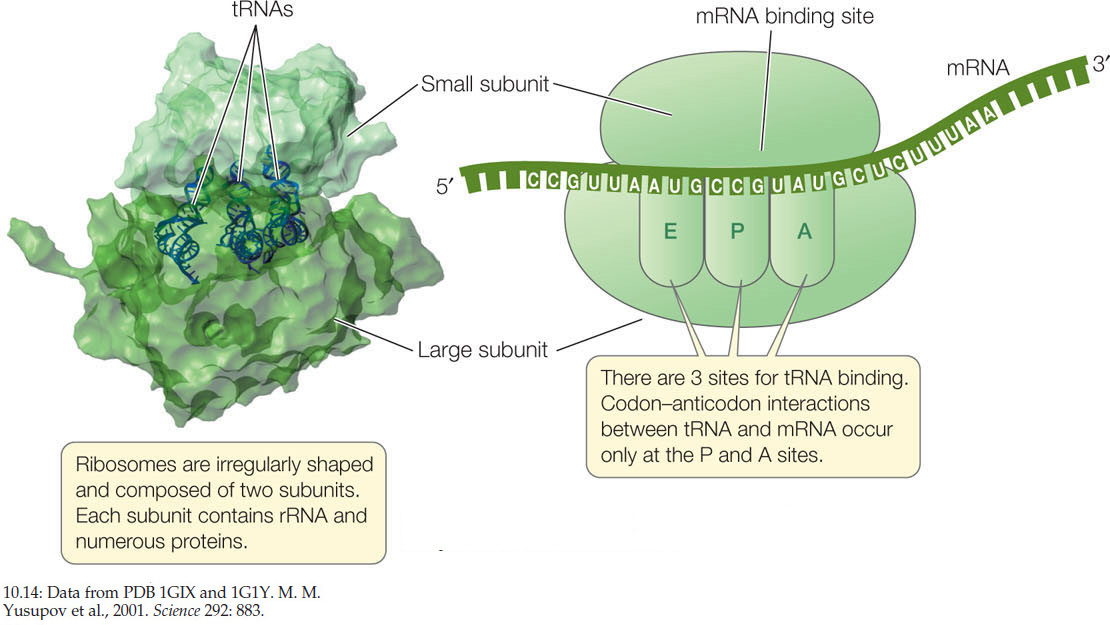
The ribosome is the molecular workbench where the translation of mRNA by tRNA is accomplished. All prokaryotic and eukaryotic ribosomes consist of two subunits (FIGURE 10.14). In eukaryotes, the large subunit consists of 3 different ribosomal RNA (rRNA) molecules and about 49 protein molecules arranged in a precise pattern. The small subunit consists of one rRNA molecule and about 33 proteins. These two subunits and several dozen other molecules interact noncovalently, fitting together like a jigsaw puzzle. If the hydrophobic interactions between the proteins and RNAs are disrupted, the ribosome falls apart, but it will reassemble perfectly when the disrupting agent is removed. When not active in the translation of mRNA, the ribosome exists as two separate subunits.
208
On the large subunit of the ribosome there are three sites to which a tRNA can bind, designated the A, P, and E sites (see Figure 10.14). The mRNA and ribosome move in relation to one another, and as they do so, a charged tRNA traverses these three sites in order:
- The A (amino acid) site is where the charged tRNA anticodon binds to the mRNA codon, thus lining up the correct amino acid to be added to the growing polypeptide chain.
- The P (polypeptide) site is where the tRNA adds its amino acid to the polypeptide chain.
- The E (exit) site is where the tRNA, having given up its amino acid, resides before being released from the ribosome and going back to the cytosol to pick up another amino acid and begin the process again.
The ribosome has a fidelity function, which ensures that a charged tRNA with the correct anticodon binds to the appropriate codon in the mRNA. When proper binding occurs, hydrogen bonds form between the three base pairs. The rRNA of the small ribosomal subunit plays a role in validating the three-base-pair match. Any tRNA that does not form hydrogen bonds with all three bases of the codon is ejected from the ribosome.
Translation takes place in three steps
Like transcription, translation occurs in three steps: initiation, elongation, and termination.
Initiation
The initiation complex consists of a charged tRNA and a small ribosomal subunit, both bound to the mRNA (FIGURE 10.15). While different organisms have different ways to effect this binding, here is an example from a prokaryote: The rRNA of the small ribosomal subunit binds by base pairing to a complementary sequence on the mRNA, about 8 base pairs upstream of the translation start codon (AUG; see Figure 10.11). After binding, the mRNA and the small subunit are aligned in such a way that the start codon at the beginning of the coding sequence will be aligned with the P site on the large subunit:
mRNA 5′ …AGGAGG … AUG …3′
rRNA 3′ … UCCUCC … (P site) … 5′
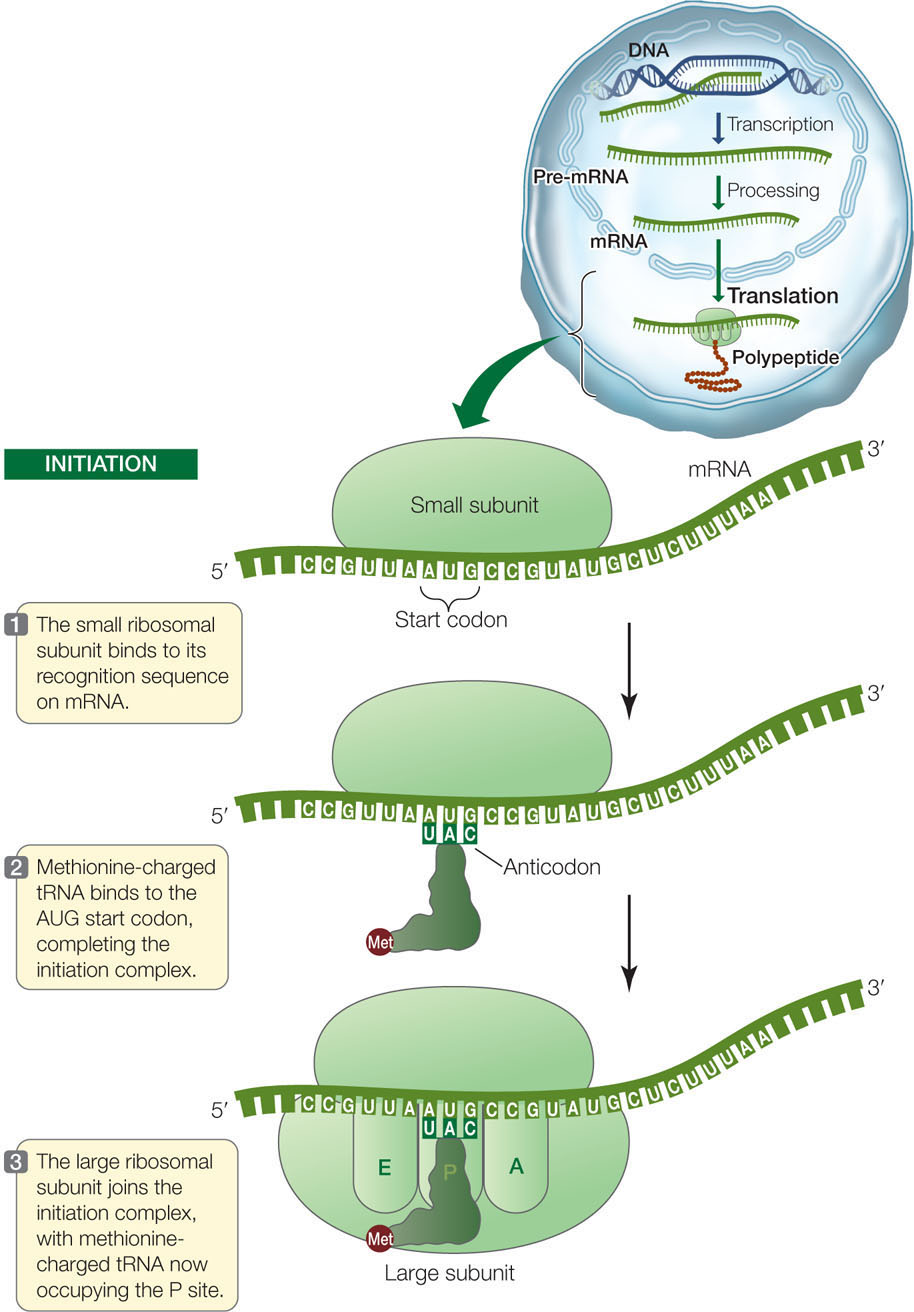
The anticodon of a methionine-charged tRNA binds to the start codon by complementary base pairing to complete the initiation complex. Thus the first amino acid in a new polypeptide chain is always methionine. (In bacteria, but not archaea, the first amino acid is a slightly modified form of methionine called formylmethionine.) However, not all mature proteins have methionine as their first amino acid. In many cases, the initial methionine is removed by an enzyme after translation.
After the methionine-charged tRNA has bound to the mRNA, the large subunit of the ribosome joins the complex. The methionine-charged tRNA lies in the P site of the large subunit, and the A site is aligned with the second mRNA codon. These ingredients—mRNA, two ribosomal subunits, and methionine-charged tRNA—are put together properly by a group of proteins called initiation factors.
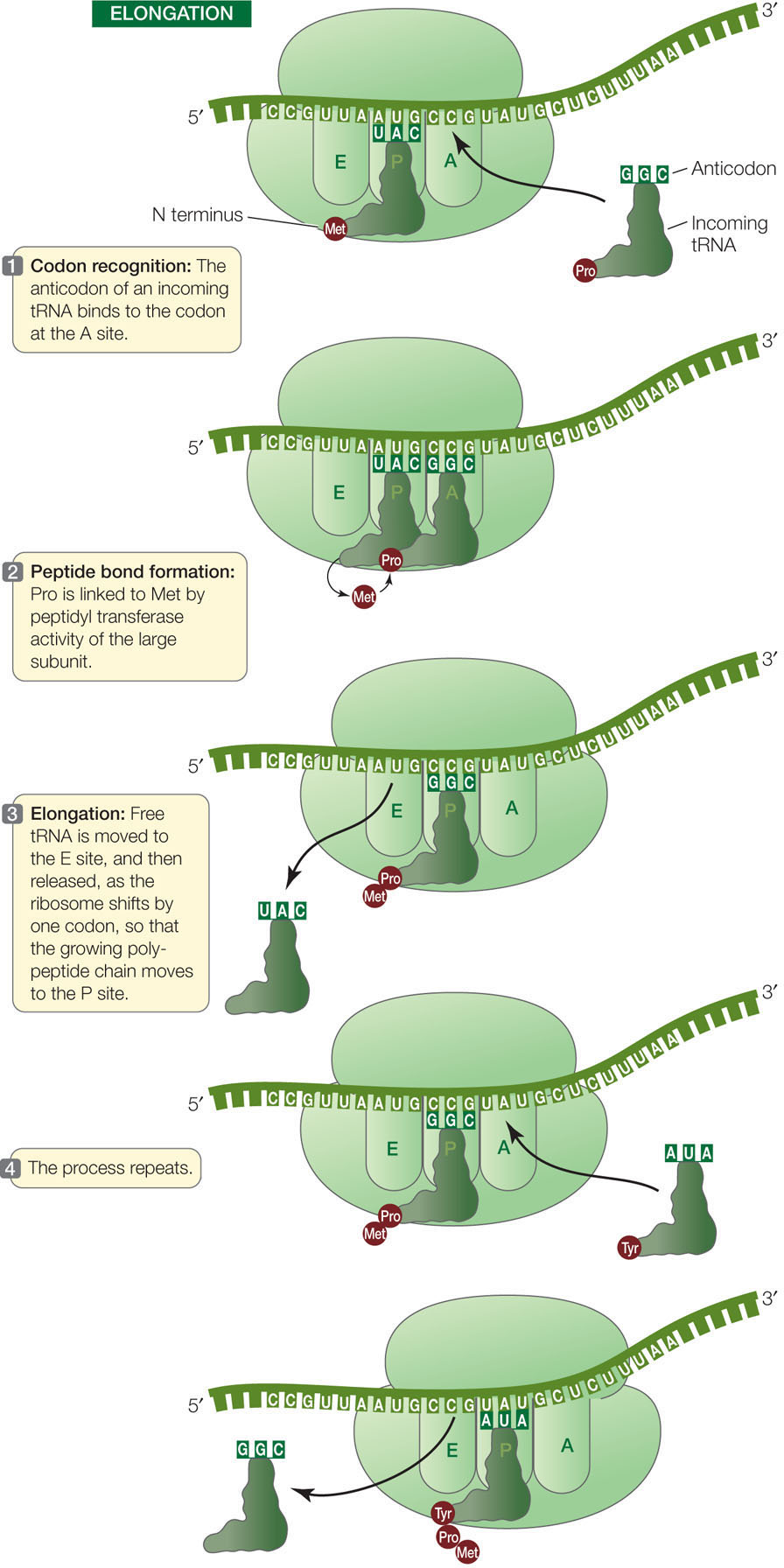
Elongation
A charged tRNA whose anticodon is complementary to the second codon of the mRNA now enters the open A site of the large ribosomal subunit (FIGURE 10.16). The large subunit then catalyzes two reactions:
- It breaks the bond between the methionine and its tRNA in the P site.
- It catalyzes the formation of a peptide bond between the methionine and the amino acid attached to the tRNA in the A site.
209
Because the large ribosomal subunit performs these two actions, it is said to have peptidyl transferase activity. The component with this activity is actually one of the rRNAs in the ribosome, so the catalyst is an example of a ribozyme (from ribonucleic acid and enzyme).
Methionine thus becomes the amino (N) terminus of the new protein (recall that polypeptides grow in the amino to the carboxyl direction; see Concept 3.2). The second amino acid is now bound to methionine but remains attached to its tRNA at the A site.
After the first tRNA releases its methionine, the ribosome moves so that the first tRNA is at the E site. The tRNA then dissociates from the ribosome and returns to the cytosol to become charged with another methionine. The second tRNA, now bearing a dipeptide (a chain of two amino acids), is shifted to the P site as the ribosome moves one codon along the mRNA in the 5′-to-3′ direction (see Figure 10.16). These steps are repeated, and the polypeptide chain grows as each new amino acid is added.
LINK
You can review the structure and formation of peptide bonds in Concept 3.2, especially Figure 3.6
Termination
The elongation cycle terminates at the end of the coding sequence, which is marked by a stop codon: UAA, UAG, or UGA (FIGURE 10.17). When a stop codon enters the A site, it binds a protein release factor, which allows hydrolysis of the bond between the polypeptide chain and the tRNA in the P site. The newly completed polypeptide then separates from the ribosome.
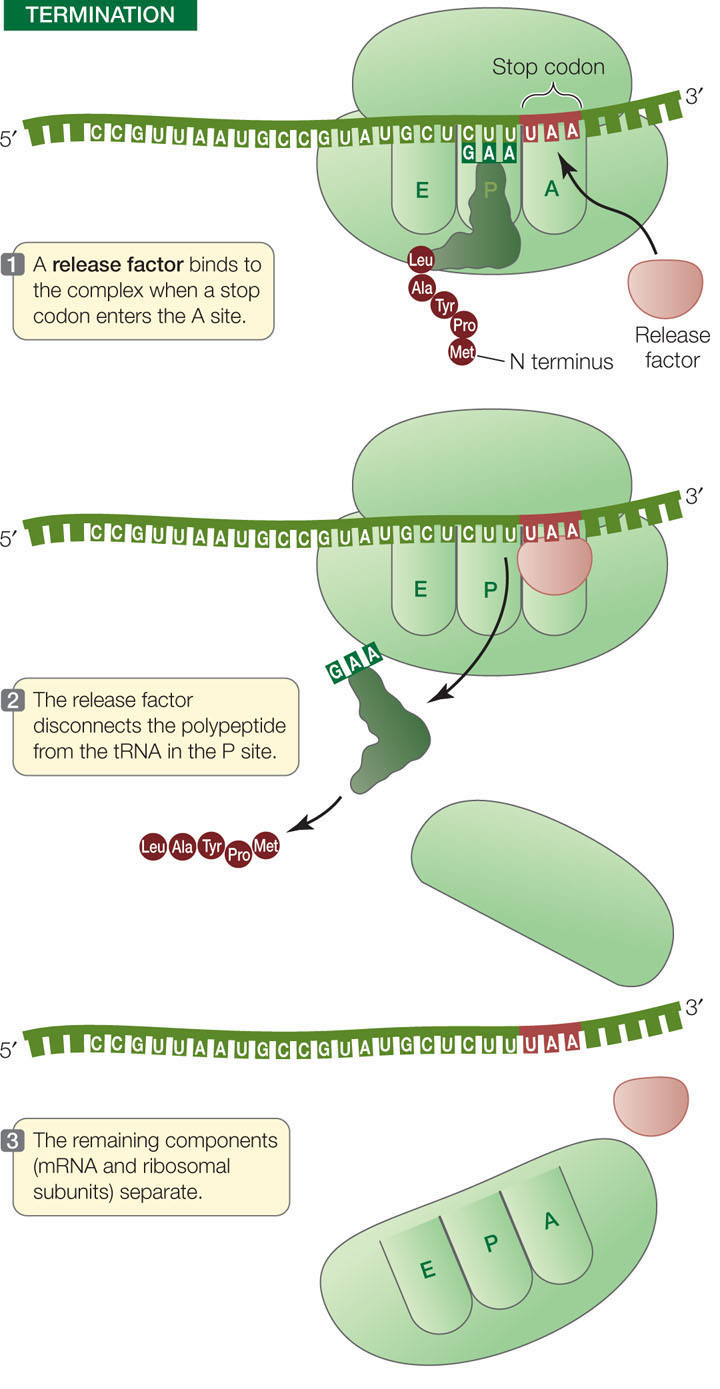
TABLE 10.2 summarizes the nucleic acid signals for initiation and termination of transcription and translation.
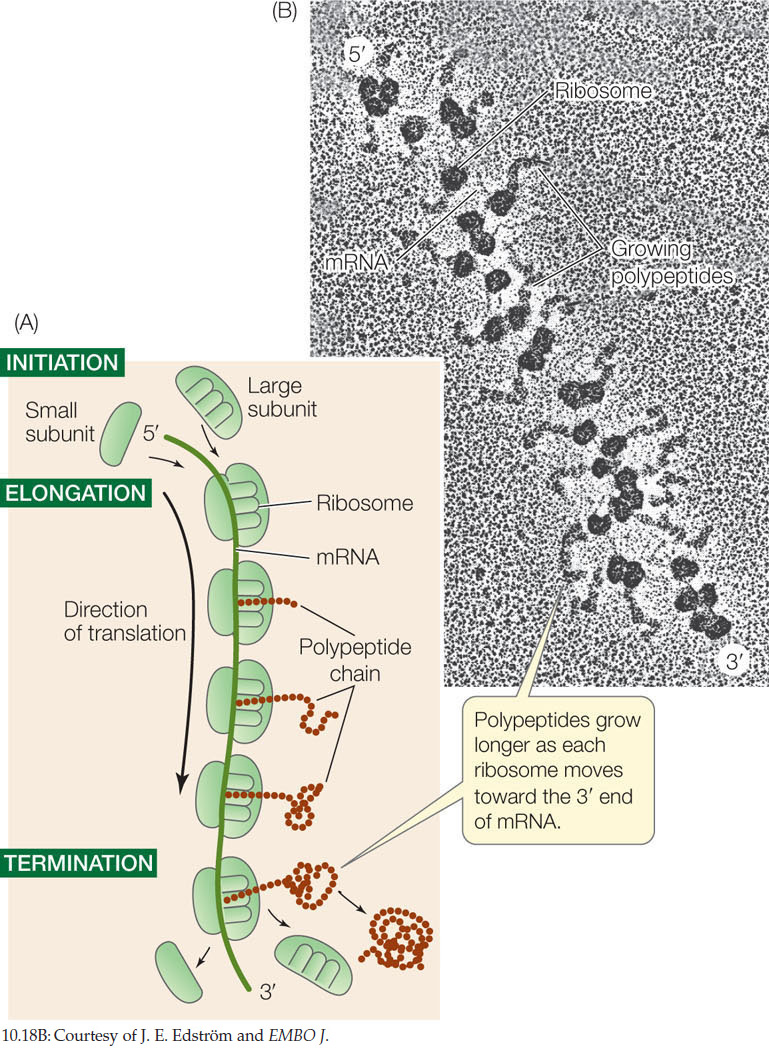
Polysome formation increases the rate of protein synthesis
Several ribosomes can simultaneously translate a single mRNA molecule, producing multiple polypeptides at the same time. As soon as the first ribosome has moved far enough from the translation initiation site, a second initiation complex can form, then a third, and so on. An assemblage consisting of a strand of mRNA with its beadlike ribosomes and their growing polypeptide chains is called a polyribosome, or polysome (FIGURE 10.18). Cells that are actively synthesizing proteins contain large numbers of polysomes and few free ribosomes or ribosomal subunits.
210
CHECKpointCONCEPT10.4
- Describe the sequence of events in translation that involve tRNA, from charging with an amino acid to initiation, elongation, and termination.
- Imagine a polypeptide whose second amino acid is tryptophan. Sketch a ribosome with the mRNA and the first two tRNAs for this polypeptide, noting their positions in the A, P, and E sites.
- What would happen to polypeptides and cell function if a valine-tRNA synthase lost its specificity and attached any of the 20 amino acids to the 3′ end of the valine tRNA?
The process of protein synthesis usually does not end with translation. Proteins can undergo covalent modifications both during and after translation, with chemical groups being added or parts of the polypeptide chains removed. We now turn to these modifications.
211