CONCEPT14.1 Development Involves Distinct but Overlapping Processes
Development is the process by which a multicellular organism, beginning with a single cell, goes through a series of changes, taking on the successive forms that characterize its life cycle (FIGURE 14.1). After the egg is fertilized it is called a zygote, and in the earliest stages of development a plant or animal is called an embryo. Progress through a series of embryonic stages precedes emergence of the new, independent organism. Many organisms continue to develop throughout their lives, with development ceasing only at death.
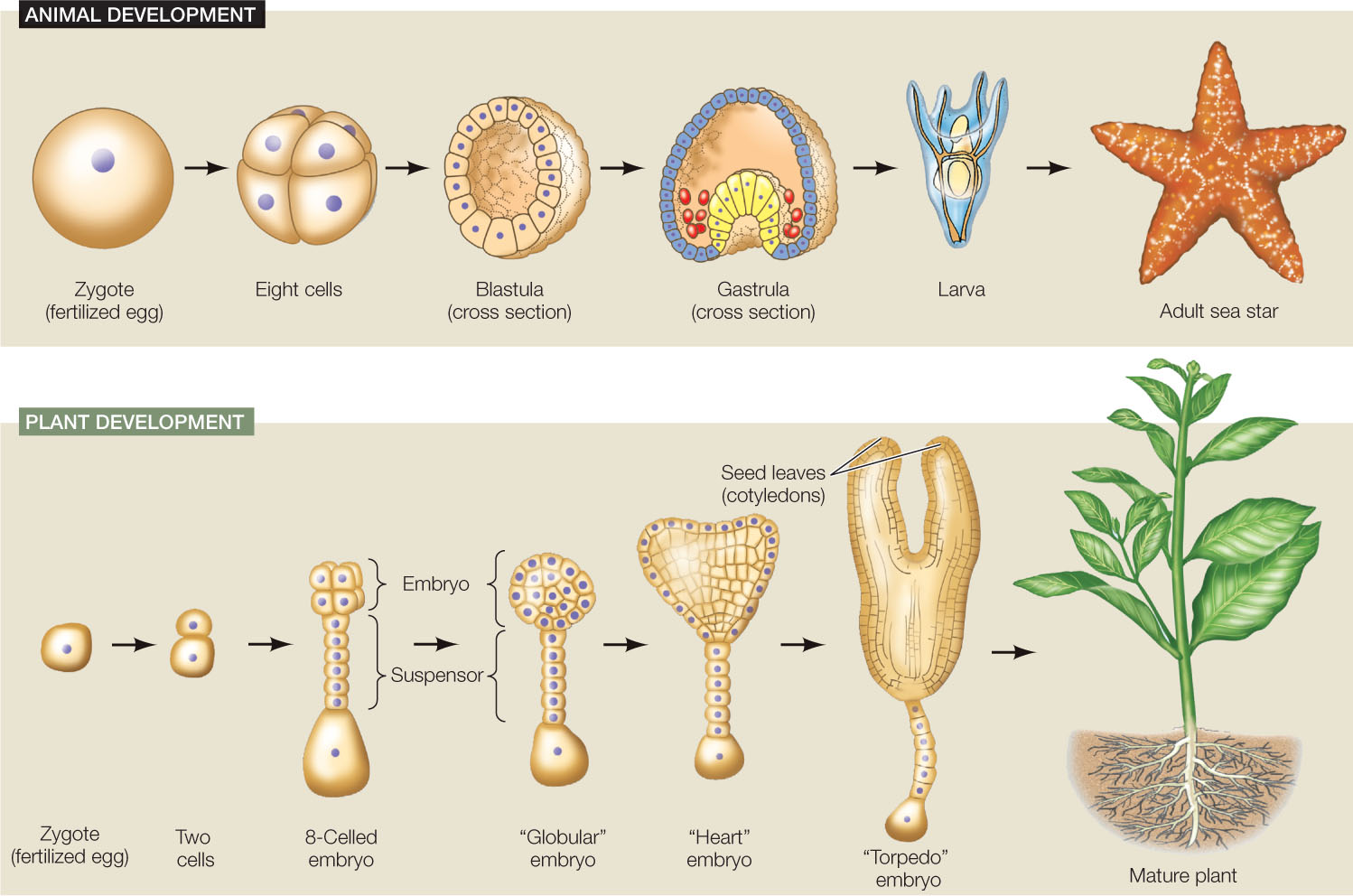
Four key processes underlie development
The developmental changes an organism undergoes as it progresses from an embryo to mature adulthood involve four processes:
- Determination sets the developmental fate of a cell—what type of cell it will become—even before any characteristics of that cell type are observable. For example, in a developing mammalian embryo, as well as in some adult organs, there are mesenchymal stem cells that look unspecialized. But their fate to become muscle, fat, tendon, or other connective tissue cells has already been determined.
- Differentiation is the process by which different types of cells arise from less specialized cells, leading to cells with specific structures and functions. For example, mesenchymal stem cells differentiate to become the cells listed above.
- Morphogenesis (Greek for “origin of form”) is the organization and spatial distribution of differentiated cells into the multicellular body and its organs. Morphogenesis can occur by cell division, cell expansion (especially in plants), cell movements, and apoptosis (programmed cell death).
- Growth is the increase in size of the body and its organs by cell division and cell expansion. Growth can occur by an increase in the number of cells or by the enlargement of existing cells. Growth continues throughout the individual’s life in some organisms, but reaches a more or less stable end point in others.
All of these processes involve differential gene expression. The cells that arise from repeated mitoses in the early embryo may look the same superficially, but they soon begin to differ in terms of which genes they express. These processes also involve signals between cells. For example, within a developing embryo there are gradients of signaling molecules called morphogens that help determine cell fate and trigger cell differentiation.
275
Cell fates become progressively more restricted during development
A zygote is a single cell that gives rise to all the cells in the organism that will develop from it. As the zygote divides to form a multicellular embryo, each of the embryo’s undifferentiated cells are destined to become part of a particular type of tissue—this is referred to as the cell fate of that undifferentiated cell.
When is the fate determined? At what point does a cell become committed to a particular fate and no other? One way to find out is to transplant cells from one embryo to a different region of a recipient embryo (FIGURE 14.2). A dye is used to mark the transplanted cells so that their subsequent development can be followed. The question is, will the transplanted cells adopt the differentiation pattern of their new surroundings, or will they continue on their own path, with their fate already sealed?
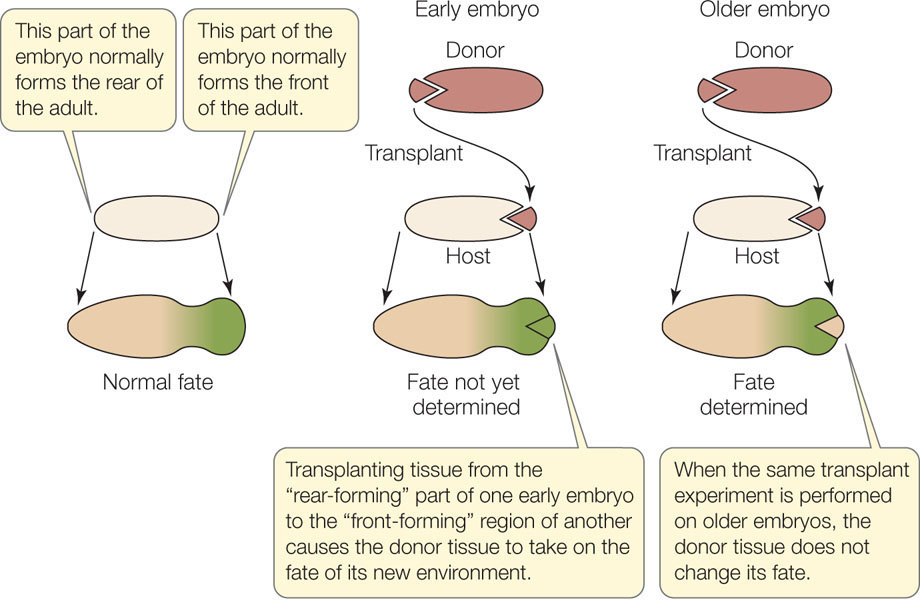
Experiments with frog embryos give an answer for that organism: If the donor tissue is from an early-stage embryo (blastula), it adopts the fate of its new surroundings; its fate has not been sealed. But if the donor tissue is from an older embryo (gastrula), it continues on its own path; its fate has been sealed. Determination is influenced by changes in gene expression as well as by the extracellular environment and is not something that is visible under the microscope—cells do not change their appearance when they become determined. Determination is followed by differentiation—the actual changes in biochemistry, structure, and function that result in cells of different types. Determination is a commitment; the final realization of that commitment is differentiation.
During animal development, cell fate becomes progressively restricted. This can be thought of in terms of cell potency, which is a cell’s potential to differentiate into other cell types:
- The cells of an early embryo are totipotent (toti, “all”; potent, “capable”); they have the potential to differentiate into any cell type, including more embryonic cells.
- In later stages of the embryo, many cells are pluripotent (pluri, “many”); they have the potential to develop into most other cell types, but they cannot form new embryos.
- Through later developmental stages, including adulthood, certain stem cells are multipotent; they can differentiate into several different, related cell types. Mesenchymal stem cells (see above) are one kind of multipotent stem cell.
- Many cells in the mature organism are unipotent; they can produce only one cell type—their own.
Cell differentiation is sometimes reversible
Once a cell’s fate is determined, the cell differentiates. However, under the right experimental conditions, a determined or differentiated cell can become undetermined again. In some cases the cell can even become totipotent, meaning it is able to form the entire organism, with all of its differentiated cells. Normally this is a property of only the zygote or, in some cases, the first few cells of the early embryo.
Plant Cell Totipotency
A carrot root cell normally faces a dark future. It cannot photosynthesize and generally does not give rise to new carrot plants. However, in 1958 Frederick Steward at Cornell University showed that if he isolated cells from a carrot root and maintained them in a suitable nutrient medium, he could induce them to dedifferentiate—to lose their differentiated characteristics. The cells could divide and give rise to masses of undifferentiated cells called calli (singular callus), which could be maintained in culture indefinitely. Furthermore, if they were provided with the right chemical cues, the cells could develop into embryos and eventually into complete new plants (FIGURE 14.3).
Investigation
HYPOTHESIS
Differentiated plant cells can be totipotent and can be induced to generate an entire new plant.
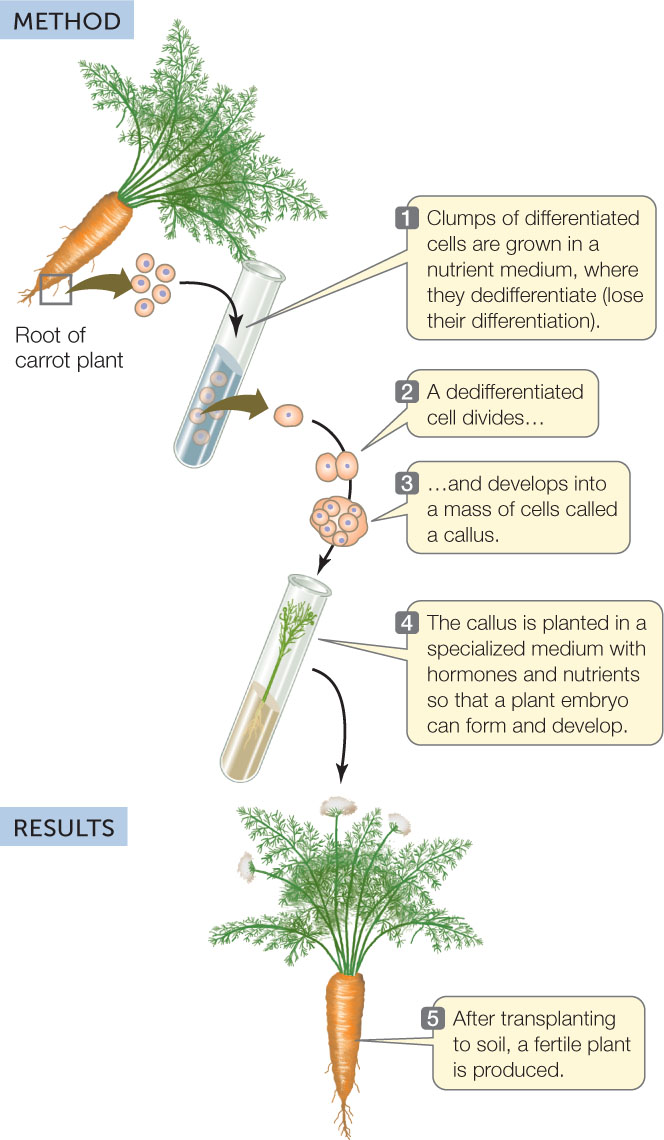
CONCLUSION
Differentiated plant cells can be totipotent.
aF. C. Steward. 1958. American Journal of Botany 45: 705-709.
Since the new plants in Steward’s experiments were genetically identical to the cells from which they came, they were clones of the original carrot plant. The ability to produce clones is evidence for the genomic equivalence of somatic (body) cells; that is, all somatic cells in a plant have a complete genome and thus have all the genetic information needed to become any cell in the plant.
Many types of cells from other plant species show similar behavior in the laboratory. This ability to generate a whole plant from groups of cells or even a single cell has been invaluable in agriculture and forestry. For example, trees from planted forests are used in making paper, lumber, and other products. To replace the trees reliably, forestry companies regenerate new trees from the leaves of selected trees with desirable traits. The characteristics of these clones are more uniform and predictable than those of trees grown from seeds.
276
Nuclear Totipotency in Animals
Animal somatic cells cannot be manipulated as easily as plant cells can. Until recently, it was not possible to induce a cell from a fully developed animal to dedifferentiate and then redifferentiate into another cell type. However, nuclear transfer experiments have shown that the genetic information from a differentiated animal cell can be used to create cloned animals. The nucleus from an unfertilized egg is removed, forming an enucleated egg. A donor nucleus from a somatic cell is then introduced into the “empty” egg. If it is then stimulated to divide, the egg forms an embryo that can develop into an adult with the genetic composition of its nuclear donor. This is the basis of cloning animals. Dolly the sheep was the first experimentally produced mammalian clone, born in 1996 (FIGURE 14.4).
Investigation
HYPOTHESIS
Differentiated animal cells are totipotent.
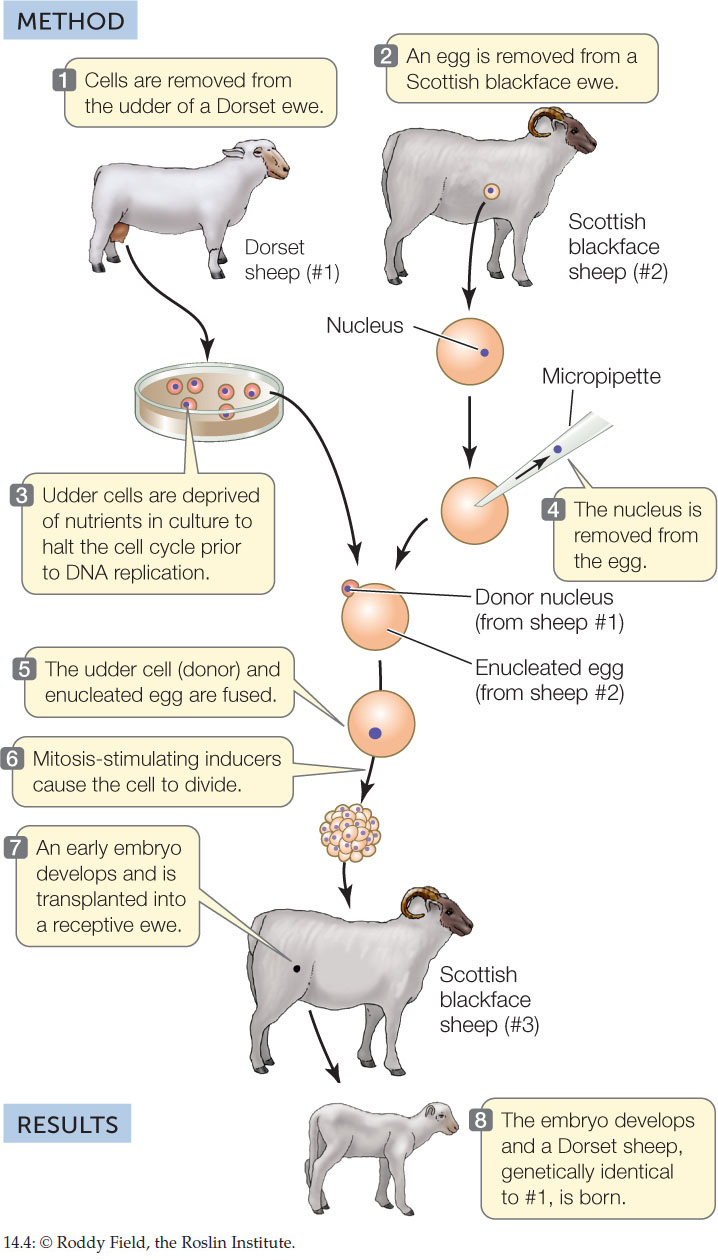
CONCLUSION
Differentiated animal cells are totipotent in nuclear transplant experiments.
ANALYZE THE DATA
The team that cloned Dolly the sheep used a nucleus from a mammary epithelium (ME) cell. They also tried cloning by transplanting nuclei from fetal fibroblasts (FB) and embryos (EC), with the results shown in the table.
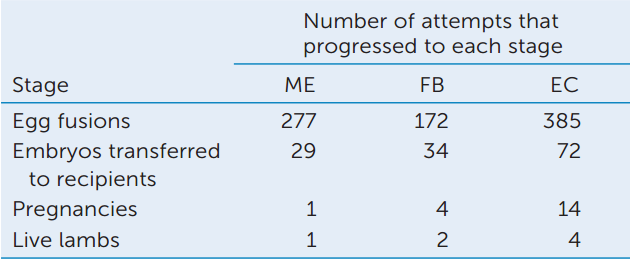
- Calculate the percentage survival of eggs from fusion to birth. What can you conclude about the efficiency of cloning?
- Compare the efficiencies of cloning using different nuclear donors. What can you conclude about the ability of nuclei at different stages to be totipotent?
- What statistical test would you use to show whether the differences in A and B were significant (see Appendix B)?

aI. Wilmut et al. 1997. Nature 385: 810-813.
Many other animal species, including cats, dogs, horses, pigs, rabbits, and mice, have since been cloned by nuclear transfer. As in plants, the cloning of animals has shown that their differentiated cells have genomic equivalence. Cloning of animals has practical uses as well:
- Expansion of the numbers of valuable animals: One goal of the researchers who produced Dolly the sheep was to develop a method of cloning transgenic animals with useful phenotypes (see Concept 13.4). For example, a cow that was genetically engineered to make human growth hormone in milk has been cloned to produce two more cows that do the same thing. Only 15 such cows could supply the world’s need for this protein, which is used to treat short stature that is due to growth hormone deficiency.
- Preservation of endangered species: The banteng, a relative of the cow, was the first endangered animal to be cloned and survive. The banteng was made using the enucleated egg from a cow, the nucleus from a banteng cell, and a cow surrogate mother. Cloning may be the only way to save endangered species with low rates of natural reproduction.
- Resurrection of extinct species. With the discoveries of intact DNA in fossils, the once-fictional idea of cloning an extinct species is becoming a possibility. In 2000, the last Pyrenian ibex—a type of mountain goat—died and the species became extinct. But in 2009, scientists used DNA from the dead animal to replace the DNA in a domestic goat egg and cloned a new ibex. Although this animal died shortly after birth, the resurrection of an extinct species was proven in principle. This has led to proposals to resurrect other extinct species, including the wooly mammoth and the Neanderthal. The genomes of both these species are available and have been sequenced.
Stem cells differentiate in response to environmental signals
The processes of development do not occur only in embryos. In adult plants, the growing regions at the tips of roots and stems contain meristems, which are clusters of undifferentiated, rapidly dividing stem cells. These cells can differentiate into the 15–20 specialized cell types that make up roots, stems, leaves, and flowers. As you will see in Chapter 26, the plant body undergoes constant growth and renewal, with new organs forming often. (Think of flowers and leaves in the spring.)
277
In adult mammals, stem cells persist in many tissues, where they are used as a pool of cells that can differentiate and replace cells that are lost by “wear and tear” (necrosis) and programmed cell death (apoptosis). This is especially evident in tissues such as the skin, inner lining of the intestine, and blood. There are about 300 different cell types in a mammal.
278
Multipotent Stem Cells
Stem cells in particular mammalian tissues are multipotent, meaning they can form a limited repertoire of differentiated cells. For example, there are two types of multipotent stem cells in bone marrow. One type (called hematopoietic stem cells) produces the various kinds of red and white blood cells. The other type (mesenchymal stem cells) produces the cells that make bone and surrounding tissues, including muscle.
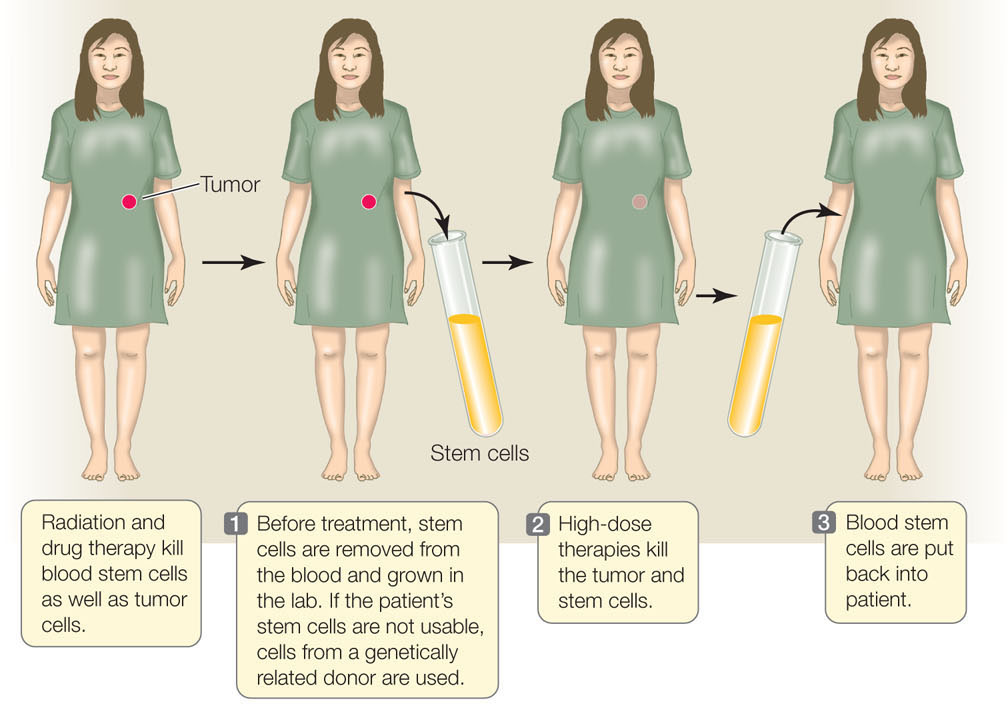
The proliferation and differentiation of multipotent stem cells is “on demand.” Hematopoeitic stem cells in the bone marrow, for example, differentiate in response to specific signals. These signals can come from either adjacent bone marrow cells or from the circulating blood. This ability to differentiate is the basis of an important cancer therapy called hematopoietic stem cell transplantation (HSCT; FIGURE 14.5). Some treatments that kill cancer cells also kill other dividing cells, including the stem cells in the bone marrow of patients exposed to these treatments. To circumvent this problem, stem cells are harvested from the blood or bone marrow of the patient prior to treatment or from a donor; the cells are injected back into the patient after cancer treatment. Before the cells are harvested, the patient (or donor) receives injections of a growth factor that stimulates proliferation of the hematopoietic stem cells. The stored stem cells retain their ability to differentiate in the bone marrow environment. By allowing the use of high doses of treatment to kill tumors, HSCT saves thousands of lives each year.
279
Pluripotent Stem Cells
In mammals, totipotent stem cells that can individually give rise to an organism are found only in very early embryos. In both mice and humans, the last embryonic stage before differentiation occurs is called a blastocyst (the term for a mammalian blastula; see Figures 14.1 and 38.8). Although they cannot form an entire embryo, a group of cells in the blastocyst still retains the ability to form any cell type in the body; these cells are pluripotent. These embryonic stem cells (ESCs) can be removed from the blastocyst and grown in laboratory culture almost indefinitely if provided with the right conditions. They can also be induced to express appropriate genes and differentiate in a particular way if the right signal is provided (FIGURE 14.6A). For example, treatment of mouse ESCs with a derivative of vitamin A causes them to form neurons (nerve cells), whereas other growth factors induce them to form blood cells. Such experiments demonstrate both the cells’ developmental potential and the roles of environmental signals. This finding raises the possibility of using ESC cultures as sources of differentiated cells to repair specific tissues, such as a damaged pancreas in diabetes, or a brain that malfunctions in Parkinson’s disease.
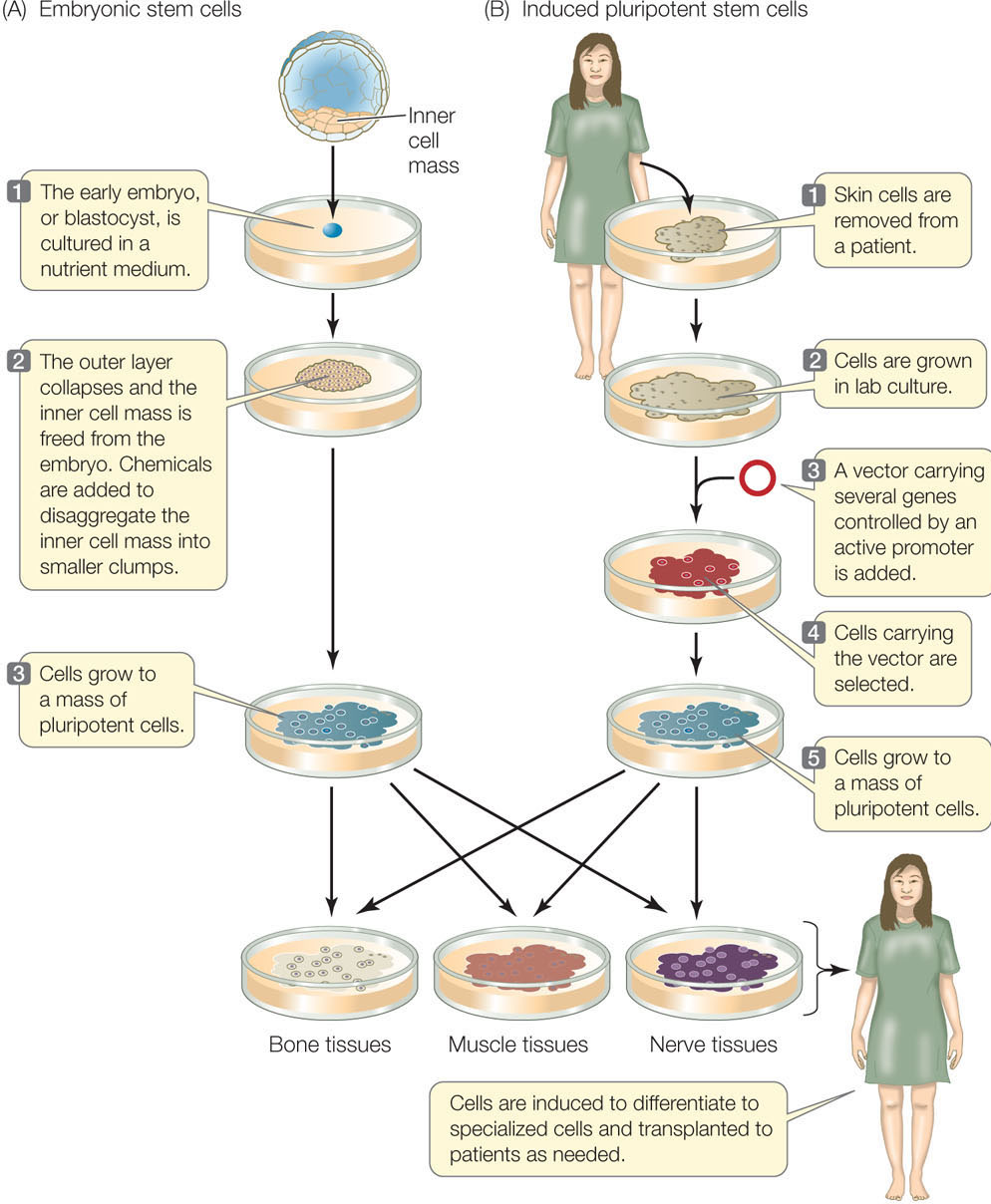
ESCs can be harvested from human embryos conceived by in vitro (“under glass”—in the laboratory) fertilization, with the consent of the donors. Since more than one embryo is usually conceived in this procedure, embryos not used for reproduction might be available for embryonic stem cell isolation. These cells could then be grown in the laboratory and used as sources of tissues for transplantation into patients with tissue damage. There are two problems with this approach:
- Some people object to the destruction of human embryos for this purpose.
- The stem cells, and tissues derived from them, would provoke an immune response in a recipient (see Chapter 39).
Shinya Yamanaka and coworkers at Kyoto University in Japan developed another way to produce pluripotent stem cells that applies the concepts of gene expression and development (FIGURE 14.6B). Instead of extracting ESCs from blastocysts, they make induced pluripotent stem cells (iPS cells) from skin cells. This approach destroys no embryos and allows tissues to be made from skin cells of any individual, thus preventing an immune response. The scientists developed this method systematically (see Chapter 13 for more information on the techniques discussed here):
- First, they used microarrays to compare the genes expressed in ESCs with those expressed in nonstem cells. They found several genes that were uniquely expressed at high levels in ESCs. These genes encode transcription factors believed to be essential to the undifferentiated state and function of stem cells. Recall that transcription factors are DNA binding proteins that regulate the expression of specific genes.
- Next, they isolated the genes and inserted them into a vector for genetic transformation of skin cells. They found that the skin cells now expressed the newly added genes at high levels.
- Finally, they showed that the transformed cells were pluripotent and could be induced to differentiate into many tissues—they had become iPS cells.
Yamanaka was awarded the Nobel Prize for his work. The ultimate aim is to use the cells for research and therapy in diseases.
CHECKpointCONCEPT14.1
- Describe the four major processes of development.
- Not all the DNA in a cell is in the nucleus. What are the genetic differences between cloning in carrot plants and cloning in sheep? How would you show this?
- Identical twins are formed when a zygote divides once by mitosis and then each mitotic product forms an embryo. Are identical twins clones? Explain your answer.
Having considered the general principles of development, we will now turn to the mechanisms that govern developmental events. Not surprisingly, these mechanisms have been studied at the molecular level and involve changes in gene expression and the activities of specific proteins.