CONCEPT14.5 Developmental Genes Contribute to Species Evolution but Also Pose Constraints
The genetic switches that allow different structures to develop in different regions of an embryo can also give rise to major morphological differences among species. We have already seen examples of this: the difference in wing number between dipterans and other insects; the development of cervical vertebrae in giraffes versus other mammals; and the differences in Gremlin expression that determine whether a bird’s foot will be webbed or not. Thus changes in the timing and position of a genetic switch can generate morphological variation, which then can be acted on by natural selection.
At the same time, the reliance of development on a genetic toolkit with a limited set of tools places constraints on how radically organisms can differ from one another. Four decades ago, the French geneticist François Jacob made the analogy that evolution works like a tinker, assembling new structures by combining and modifying the available materials, and not like an engineer, who is free to develop dramatically different designs (say, a jet engine to replace a propeller-driven engine). The evolution of morphology has not been governed by the appearance of radically new genes, but by modifications of existing genes and their regulatory pathways. Thus developmental genes and their expression constrain evolution in two major ways:
- Nearly all evolutionary innovations are modifications of previously existing structures.
- The genes that control development are highly conserved; that is, the regulatory genes usually change slowly over the course of evolution.
Mutations in developmental genes can cause major morphological changes
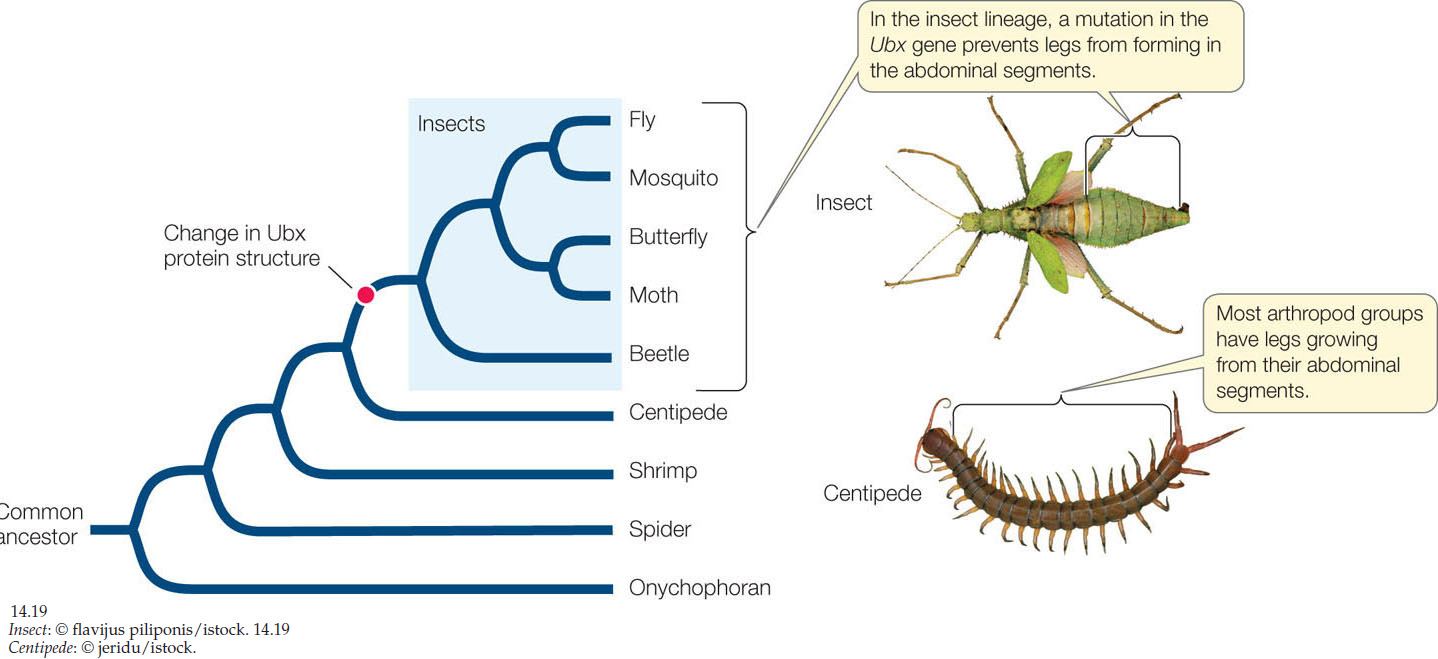
Sometimes a major developmental change is due to an alteration in the regulatory molecule itself rather than a change in where, when, or how much it is expressed. This is called heterotypy (“different type”). An excellent example of heterotypy is a gene that controls the number of legs in arthropods. Arthropods all have head, thoracic, and abdominal regions with variable numbers of segments. Insects such as Drosophila have three pairs of legs, one pair on each of their three thoracic segments, whereas centipedes have many legs on both thoracic and abdominal segments. All arthropods express a gene called Distalless (Dll) that controls segmental leg development. In insects, Dll expression is repressed in abdominal segments by the Hox gene Ubx. Ubx is expressed in the abdominal segments of all arthropods, but it has different effects in different species. In centipedes, Ubx protein activates expression of the Dll gene to promote the formation of legs. During the evolution of insects, a change in the Ubx gene sequence resulted in a modified Ubx protein that represses Dll expression in abdominal segments. A phylogenetic tree of arthropods shows that this change in Ubx occurred in the ancestor of insects, at the same time that abdominal legs were lost (FIGURE 14.19).
292
LINK
Arthropod evolution and diversity are discussed in Concept 23.4
Evolution proceeds by changing what’s already there
The features of organisms almost always evolve from preexisting features in their ancestors. New “wing genes” did not suddenly appear in birds and bats; instead, wings arose as modifications of existing structures (FIGURE 14.20). In vertebrates, the wings are modified limbs.
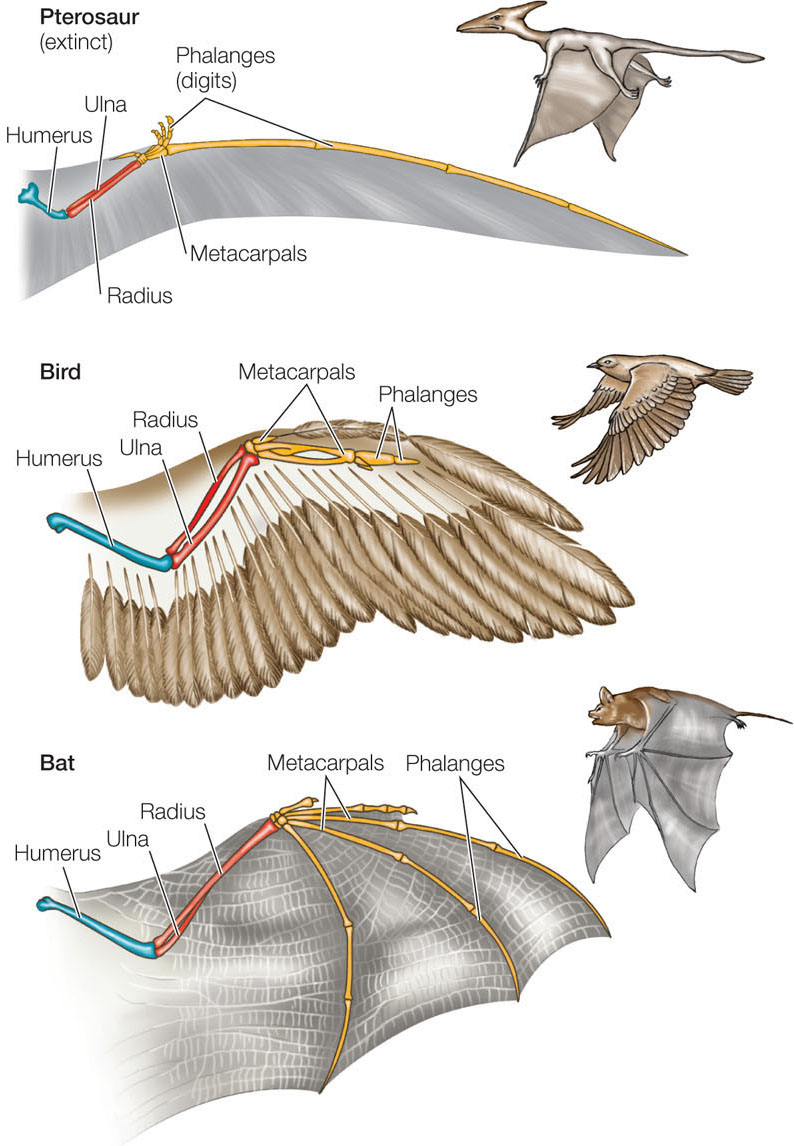
Although the wings of birds and bats look different, they are made from the same basic parts. Like limbs, wings have a common structure: a humerus that connects to the body; two longer bones, the radius and ulna, that project away from the humerus; and then metacarpals and phalanges (digits). During development these bones take on different lengths and weights in different organisms. For example, the phalanges are relatively short in birds and relatively long in bats. These differences arise from changes in the molecular mechanisms that control development, as we saw for cervical vertebrae in giraffes.
293
Developmental controls also influence how organisms lose structures. The ancestors of present-day snakes lost their forelimbs as a result of changes in the segmental expression of Hox genes. The snake lineage subsequently lost its hindlimbs by the loss of expression of the Sonic hedgehog gene in the limb bud tissue. But some snake species such as boas and pythons still have rudimentary pelvic bones and upper leg bones. Recall that Sonic hedgehog also functions as a morphogen in hand development. This is yet another example of how the same basic genetic tools are used in different ways in different species.
Conserved developmental genes can lead to parallel evolution
As we saw for the Hox genes, the nucleotide sequences of many developmental genes have been highly conserved throughout the evolution of multicellular organisms. In other words, these genes exist in similar form across a broad spectrum of species. The existence of these highly conserved genes makes it likely that similar traits will evolve repeatedly, especially among closely related species, in a phenomenon called parallel phenotypic evolution. A good example is provided by a small fish, the three-spined stickleback (Gasterosteus aculeatus: “bony stomach with spines”).
Sticklebacks are widely distributed across the Atlantic and Pacific oceans and are also found in many freshwater lakes. Marine populations of this species spend most of their lives at sea but return to fresh water to breed. Members of freshwater populations live in lakes and never journey to salt water. Genetic evidence shows that freshwater populations have arisen independently from marine populations many times in different parts of the world, most recently at the end of the last ice age. Marine sticklebacks have structures that protect them from predators: well-developed pelvic bones with pelvic spines, and bony plates. In each of the separate freshwater populations, this body armor is greatly reduced, and dorsal and pelvic spines are much shorter or even lacking (FIGURE 14.21).
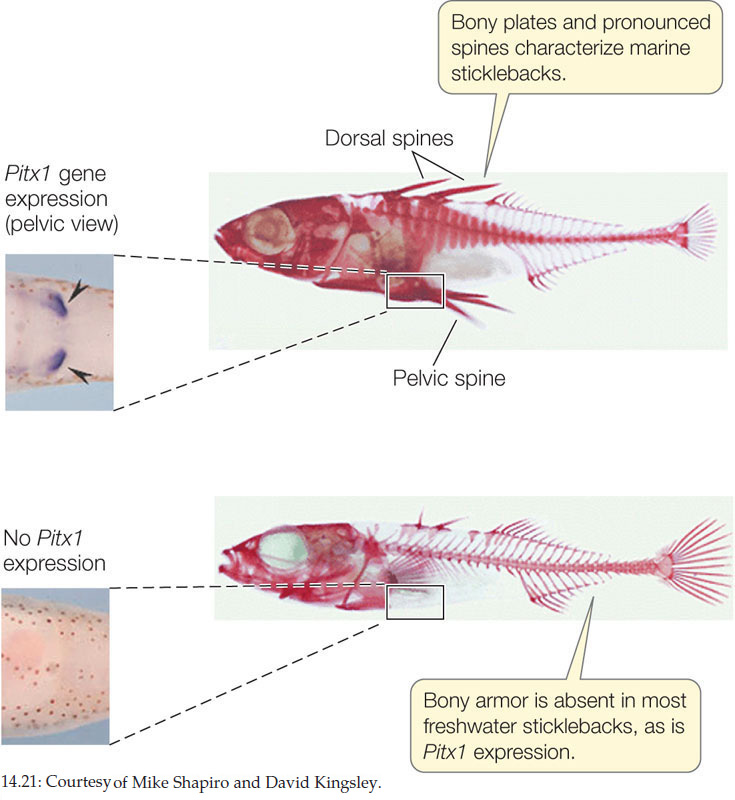
The difference between marine and freshwater sticklebacks is not induced by environmental conditions. Marine species that are reared in fresh water still grow spines. Not surprisingly, the difference is due to a gene that affects development. The Pituitary homeobox transcription factor 1 (Pitx1) gene codes for a transcription factor that is normally expressed in regions of the developing embryo that form the head, trunk, tail, and pelvis of the marine stickleback. However, in independent freshwater populations from Canada, the United Kingdom, the United States, and Iceland, the gene is no longer expressed in the pelvis, and the spines do not develop. This same gene sequence has evolved to produce similar phenotypic changes in several independent populations, and is thus a good example of parallel evolution. What could be the common selective mechanism in these cases? Possibly, the decreased predation pressure in the freshwater environment allows for increased reproductive success in animals that invest less energy in the development of unnecessary protective structures.
CHECKpointCONCEPT14.5
- How have diverse body forms such as wings evolved by means of modifications in the functioning of existing genes?
- What would happen at the molecular and phenotypic levels if a Ubx gene from an adult insect replaced the Ubx gene in a fertilized insect egg?
- When several freshwater populations of stickleback fish were compared, the coding region of the Pitx1 gene was identical to that found in marine populations. But in every case, the freshwater fish had mutations in noncoding regions of Pitx1 that led to reduced expression. What might these noncoding-region mutations be?
294
How do gene products control the development of the eye?
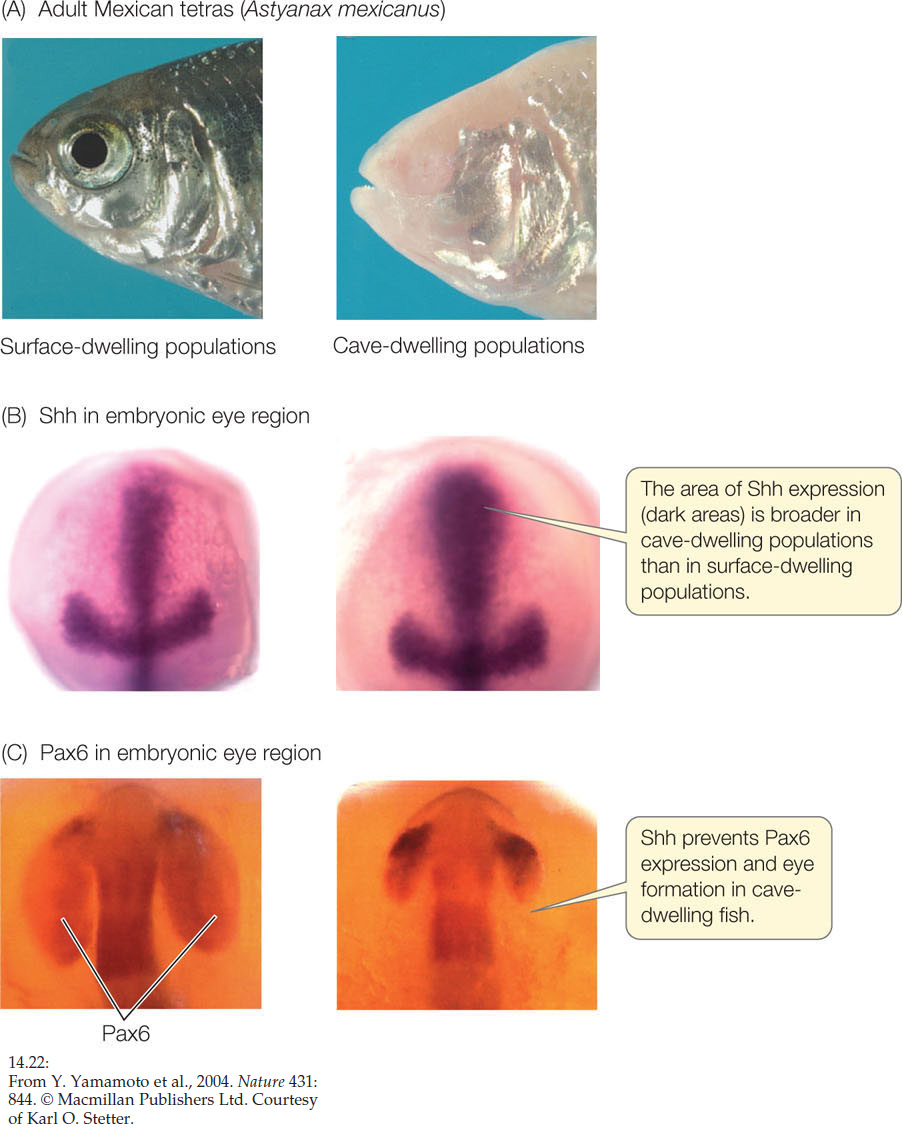
ANSWER After reading this chapter, it should not surprise you that the product of both the fruit fly eyeless gene and the vertebrate Pax6 gene is a transcription factor. This protein is produced in the front of the developing brain in a region called the neural plate. The result is a region called the “eye field,” where an eye can develop. The separation of this single region into two eyes occurs when cells in the middle of the region produce Shh (Sonic hedgehog—a protein that is also involved in limb specification). Shh is a transcription factor that blocks the synthesis of Pax6, so where there is Shh, there is no eye development. Typically Shh is produced only in a central region of the neural plate. But in cave-dwelling fish, it occurs over a wider area of the eye field and adults have no eyes (FIGURE 14.22). If too little or no Shh is made, a single eye is formed; this occurs in the human disorder known as cyclopia.
295