CONCEPT23.5 Deuterostomes Include Echinoderms, Hemichordates, and Chordates
It may surprise you to learn that you and a sea urchin are both deuterostomes. Adult sea stars, sea urchins, and sea cucumbers—the most familiar echinoderms—look so different from adult vertebrates (fishes, frogs, lizards, birds, and mammals) that it is difficult to believe all these animals are closely related. Two major pieces of evidence indicate that the deuterostomes share a common ancestor not shared with the protostomes:
- Deuterostomes share a pattern of early development in which the mouth forms at the opposite end of the embryo from the blastopore, and the blastopore develops into the anus (this is opposed to the protostomes, in which the blastopore becomes the mouth).
- Recent phylogenetic analyses of the DNA sequences of many different genes offer strong support for the shared evolutionary relationships of deuterostomes.
Note that neither of the above factors is apparent in the morphology of the adult animals.
498
Although there are far fewer species of deuterostomes than of protostomes (see Table 23.1), the deuterostomes are of special interest because they include many large animals—including humans—that strongly influence the characteristics of ecosystems. Many deuterostome species have been intensively studied in all fields of biology. Complex behaviors are especially well developed among some deuterostomes and are a vast and fascinating field of study in themselves (see Chapter 40).
There are three major clades of living deuterostomes:
- Echinoderms: sea stars, sea urchins, and their relatives
- Hemichordates: acorn worms and pterobranchs
- Chordates: sea squirts, lancelets, and vertebrates
All deuterostomes are triploblastic and coelomate (see Figure 23.4C). Skeletal support features, where present, are internal rather than external. Some species have segmented bodies, but the segments are less obvious than those of annelids and arthropods.
The earliest deuterostomes were bilaterally symmetrical, segmented animals with a pharynx that had slits through which water flowed. Echinoderms evolved their adult forms with unique symmetry (in which the body parts are arranged along five radial axes) much later, whereas other deuterostomes retained the ancestral bilateral symmetry.
The echinoderms and hemichordates (together known as ambulacrarians) have a bilaterally symmetrical, ciliated larva (FIGURE 23.32A). Adult hemichordates also are bilaterally symmetrical. Echinoderms, however, undergo a radical change in form as they develop into adults (FIGURE 23.32B), changing from a bilaterally symmetrical larva to an adult with pentaradial symmetry (symmetry in five or multiples of five). As is typical of animals with radial symmetry, echinoderms have no head, and they move equally well (but usually slowly) in many directions. Rather than having an anterior–posterior (head–tail) and dorsal–ventral (back–belly) body organization, echinoderms have an oral side (containing the mouth) and an opposite aboral side (containing the anus).
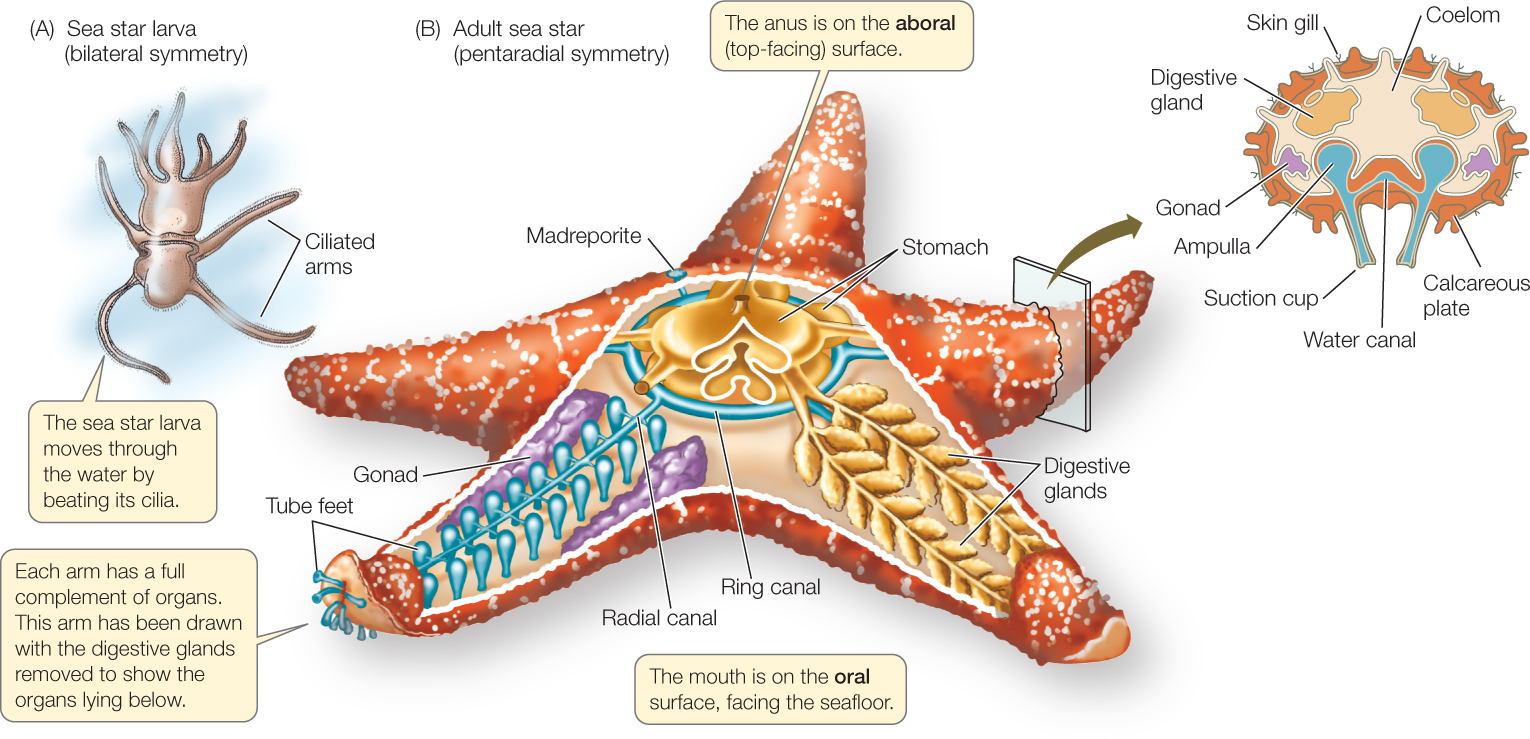
Echinoderms have unique structural features
About 13,000 species of echinoderms in 23 major groups have been described from fossil remains. They are probably only a small fraction of those that actually lived. Only 6 of the 23 major groups known from fossils are represented by species that survive today. Many clades of echinoderms became extinct during the periodic mass extinctions that have occurred throughout Earth’s history (see Table 18.1). Nearly all of the 7,500 extant species of echinoderms live only in marine environments.
In addition to having pentaradial symmetry, adult echinoderms have two unique structural features. One is a system of calcified internal plates covered by thin layers of skin and some muscles. The calcified plates of most echinoderms are thick, and they fuse inside the entire body, forming an internal skeleton. The other unique feature is a water vascular system, a network of water-filled canals leading to extensions called tube feet. This system functions in gas exchange, locomotion, and feeding (see Figure 23.32B).
499
Members of one major extant clade, the crinoids (sea lilies and feather stars), were more abundant and species-rich 300–500 million years ago than they are today. There are some 80 described living sea lily species, most of which are sessile organisms attached to a substrate by a stalk. Feather stars (FIGURE 23.33A) grasp the substrate with flexible appendages that allow for limited movement. About 600 living species of feather stars have been described.

Unlike the crinoids, most of the other surviving echinoderms are motile. The two main groups of motile echinoderms are the echinozoans (sea urchins and sea cucumbers; FIGURE 23.33B,C) and asterozoans (sea stars and brittle stars; FIGURE 23.33D,E).
The tube feet of the different echinoderm groups have been modified in a great variety of ways to capture prey. Sea lilies, for example, feed by orienting their arms in passing water currents. Food particles strike and stick to their tube feet, which are covered with mucus-secreting glands. The tube feet transfer food particles to grooves in the arms, where ciliary action carries the particles to the mouth. Sea cucumbers capture food with their anterior tube feet, which are modified into large, feathery, sticky tentacles that can be protruded from the mouth. Periodically, a sea cucumber withdraws the tentacles, wipes off the material that has adhered to them, and digests it.
Many sea stars use their tube feet to capture large prey such as annelids, gastropod and bivalve mollusks, small crustaceans such as crabs, and fishes. With hundreds of tube feet acting simultaneously, a sea star can grasp a bivalve in its arms, anchor the arms with its tube feet, and by steady contraction of the muscles in its arms, gradually exhaust the muscles the bivalve uses to keep its shell closed (see Figure 23.33D). To feed on a bivalve, a sea star can push its stomach out through its mouth and then through the narrow space between the two halves of the bivalve’s shell. The sea star’s stomach then secretes enzymes that digest the prey.
Most sea urchins eat algae, which they catch with their tube feet from the plankton or scrape from rocks with a complex rasping structure. Most of the 2,000 species of brittle stars ingest particles from the upper layers of sediments and assimilate the organic material from them, although some species filter suspended food particles from the water, and others capture small animals.
Hemichordates are wormlike marine deuterostomes
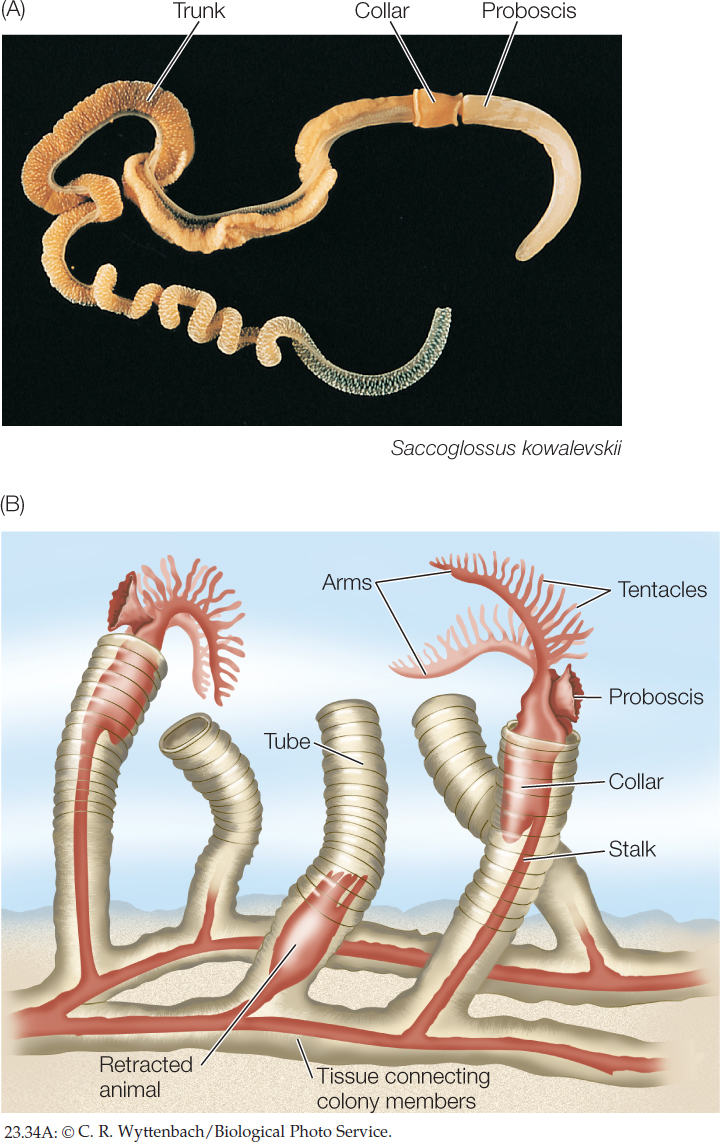
The roughly 120 species of hemichordates—acorn worms and pterobranchs—have a body organized in three major parts, consisting of a proboscis, a collar (which bears the mouth), and a trunk (which contains the other body parts). The 90 known species of acorn worms range up to 2 meters in length (FIGURE 23.34A). They live in burrows in muddy and sandy marine sediments. The digestive tract of an acorn worm consists of a mouth behind which are a muscular pharynx and an intestine. The pharynx opens to the outside through a number of pharyngeal slits through which water can exit. Highly vascularized tissue surrounding the pharyngeal slits serves as a gas exchange apparatus. Acorn worms breathe by pumping water into the mouth and out through the pharyngeal slits. They capture prey with the large proboscis, which is coated with sticky mucus to which small organisms in the sediment stick. The mucus and its attached prey are conveyed by cilia to the mouth. In the esophagus, the food-laden mucus is compacted into a ropelike mass that is moved through the digestive tract by ciliary action.
500
The 30 living species of pterobranchs are sedentary marine animals up to 12 millimeters long that live in a tube secreted by the proboscis. Some species are solitary; others form colonies of individuals joined together (FIGURE 23.34B). Behind the proboscis is a collar with anywhere from one to nine pairs of arms. The arms bear long tentacles that capture prey and function in gas exchange.
Chordate characteristics are most evident in larvae
As mentioned earlier, it is not obvious from examining the morphology of adult animals that echinoderms and chordates share a common ancestor. The evolutionary relationships among some chordate groups are not immediately apparent either. The features that reveal these evolutionary relationships are seen primarily in the larvae—in other words, it is during the early developmental stages that their evolutionary relationships are evident.
There are three principal chordate clades: the lancelets (also called cephalochordates), tunicates (also called urochordates), and vertebrates. Adult chordates vary greatly in form, but all chordates display the following derived structures at some stage in their development (FIGURE 23.35):
- A dorsal hollow nerve cord
- A tail that extends beyond the anus
- A dorsal supporting rod called the notochord
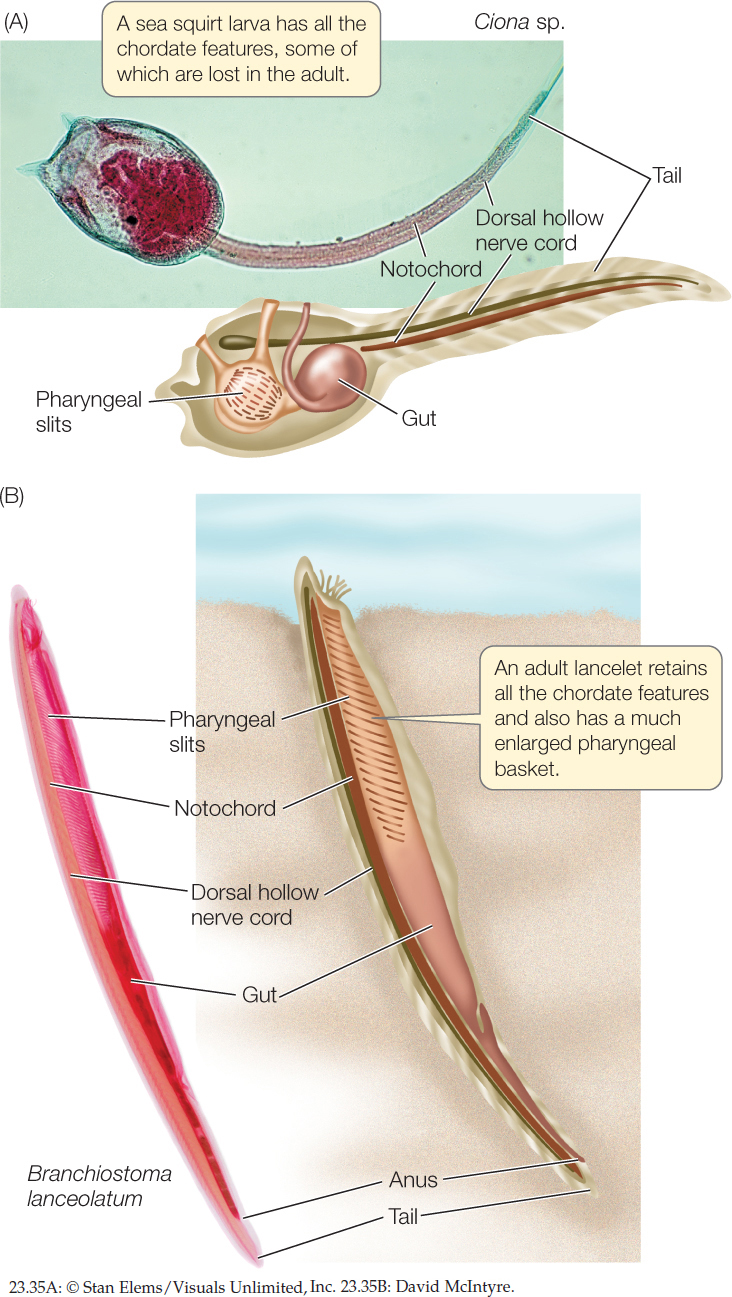
The notochord is the most distinctive derived chordate trait. It is composed of a core of large cells with turgid fluid-filled vacuoles, which make it rigid but flexible. In most tunicates the notochord is lost during metamorphosis to the adult stage. In most vertebrate species, it is replaced during development by vertebrae that provide support for the body.
The ancestral pharyngeal slits (not a derived feature of this group) are present at some developmental stage of chordates but are often lost in adults. The pharynx, which develops around the pharyngeal slits, functioned in chordate ancestors as the site for oxygen uptake and the elimination of carbon dioxide and water (as in acorn worms). The pharynx is much enlarged in some chordate species (as in the pharyngeal basket of the lancelet in Figure 23.35B).
Adults of most lancelets and tunicates are sessile
The 35 species of lancelets are small animals that rarely exceed 5 centimeters in length. The notochord, which provides body support, extends the entire length of the body throughout their lives (see Figure 23.35B). Lancelets are found in shallow marine and brackish waters worldwide. Most of the time they lie covered in sand with their head protruding above the sediment, but they can swim. They filter prey from the water with their pharyngeal basket.
All members of the three major tunicate groups—the sea squirts, salps, and larvaceans—live in marine environments. More than 90 percent of the 2,800 known species of tunicates are sea squirts (also called ascidians). Individual sea squirts range in length from less than 1 millimeter to 60 centimeters. Some sea squirts form colonies by asexual budding from a single founder. Colonies may measure several meters across. The baglike body of an adult sea squirt is enclosed in a tough tunic, which is the basis for the name “tunicate” (FIGURE 23.36). The tunic is composed of proteins and a complex polysaccharide secreted by epidermal cells. The sea squirt pharynx is enlarged into a pharyngeal basket that filters prey from the water passing through it.

501
In addition to its pharyngeal slits, a sea squirt larva has a dorsal hollow nerve cord and a notochord that is restricted mostly to the tail region (see Figure 23.35A). Bands of muscle that surround the notochord provide support for the body. After a short time swimming in the plankton, the larvae of most species settle on the seafloor and transform into sessile adults. The swimming, tadpolelike larvae suggest a close evolutionary relationship between sea squirts and vertebrates (see Figure 16.4).
As we have discussed, the hemichordates, echinoderms, lancelets, and tunicates are marine animals. The lineage that led to the vertebrates is also thought to have evolved in the oceans, although probably in an estuarine environment (where fresh water meets salt water). Vertebrates have since radiated into marine, freshwater, terrestrial, and aerial environments worldwide.
The vertebrate body plan can support large, active animals
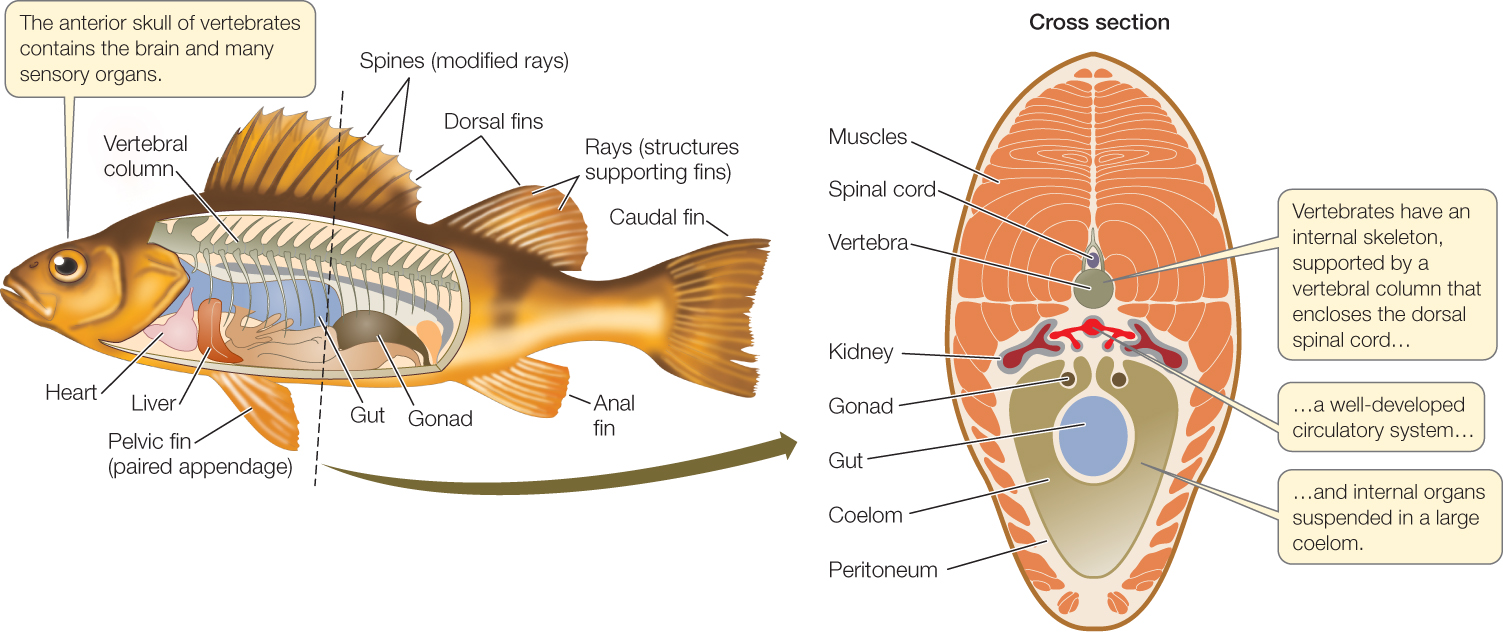
The vertebrates take their name from the unique, jointed, dorsal vertebral column that replaces the notochord during early development as their primary supporting structure. The individual elements in the vertebral column are called vertebrae. Four other key features characterize the vertebrates as well (FIGURE 23.37):
- An anterior skull with a large brain
- A rigid internal skeleton supported by the vertebral column, which also encloses the spinal cord
- Internal organs suspended in a coelom
- A well-developed circulatory system, driven by contractions of a ventral heart
The internal skeleton provides support for an extensive muscular system, which receives oxygen from the circulatory system and is controlled by the central nervous system. The evolution of these features allowed many vertebrates to become large, active predators, which in turn allowed the vertebrates to diversify widely (FIGURE 23.38).
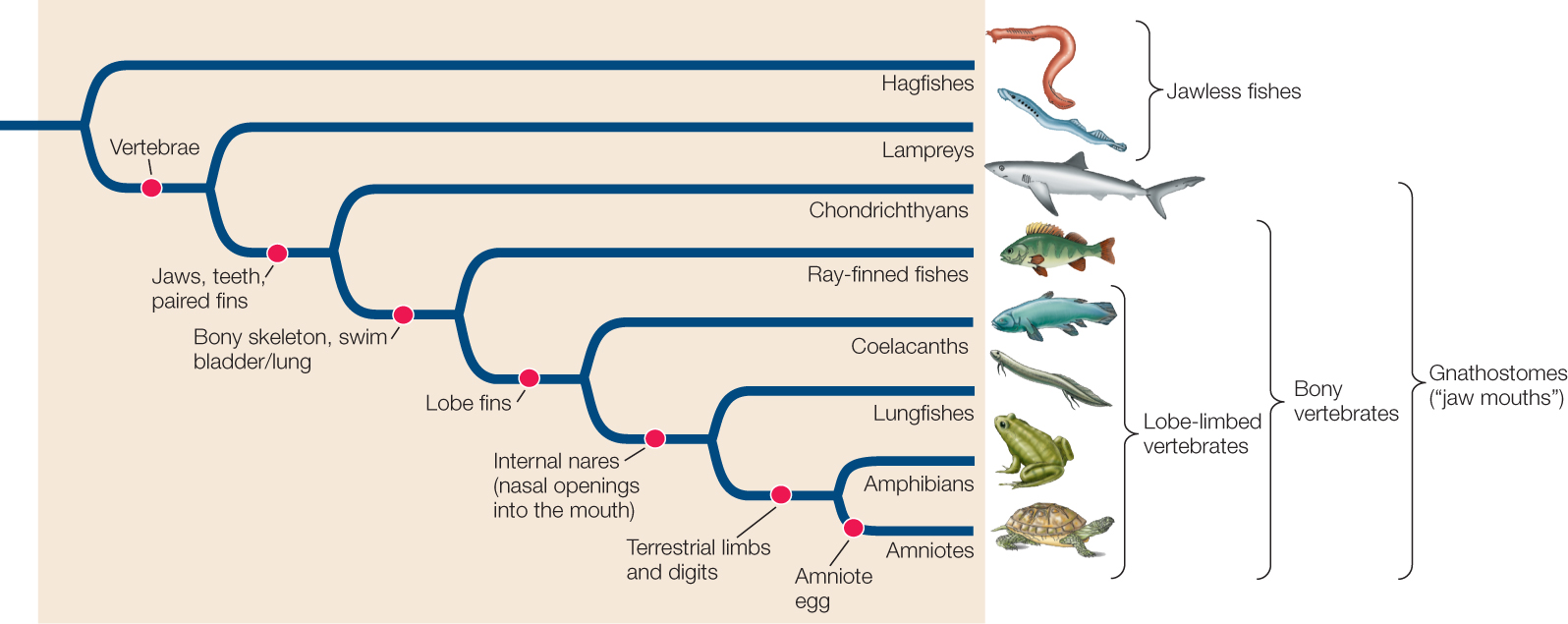
There are two groups of living jawless fishes
The hagfishes are thought by many biologists to be the sister group to the remaining vertebrates (see Figure 23.38). Hagfishes (FIGURE 23.39A) have a weak circulatory system, with three small accessory hearts (rather than a single, large heart), a partial cranium or skull (containing no cerebrum or cerebellum, two main regions of the brain of other vertebrates), and no jaws or stomach. They also lack separate, jointed vertebrae and have a skeleton composed of cartilage. Thus some biologists do not consider hagfishes vertebrates, and use instead the term “craniates” to refer collectively to the hagfishes and the vertebrates. Some analyses of gene sequences suggest, however, that hagfishes may be more closely related to the vertebrate lampreys (FIGURE 23.39B); in this phylogenetic arrangement, the two groups are collectively called the cyclostomes (“circle mouths”). If in fact the hagfishes and lampreys do form a monophyletic group, then hagfishes must have secondarily lost many of the major vertebrate morphological features during their evolution.

502
The 80 known species of hagfishes are unusual marine animals that produce copious quantities of slime as a defense. They are virtually blind and rely largely on the four pairs of sensory tentacles around their mouth to detect food. Although they have no jaws, hagfishes have a tonguelike structure equipped with toothlike rasps that they can use to tear apart dead organisms and to capture their principal prey, annelid worms. Hagfishes have direct development (no larvae), and individuals may actually change sex from year to year (from male to female and vice versa).
503
Although the lampreys and hagfishes may look superficially similar (with elongate eel-like bodies and no paired fins), they differ greatly in their biology. Lampreys have a complete skull and distinct and separate (although rudimentary) vertebrae, all cartilaginous rather than bony. Lampreys undergo a complete metamorphosis from filter-feeding larvae known as ammocoetes, which are morphologically quite similar in general structure to adult lancelets. The adults of many species of lampreys are parasitic, although several lineages of lampreys evolved to become nonfeeding as adults. These nonfeeding adult lampreys survive only a few weeks after metamorphosis—just long enough to breed. In the species that are parasitic as adults, the round mouth is a rasping and sucking organ that is used to attach to their prey and rasp at the flesh (see Figure 23.39B).
The nearly 50 species of lampreys either live in fresh water or live in coastal salt water and move into fresh water to breed. Some species of lampreys are critically endangered because of recent habitat changes and losses.
Jaws and teeth improved feeding efficiency
Many kinds of jawless fishes were found in the seas, estuaries, and fresh waters of the Devonian period, but hagfishes and lampreys are the only jawless fishes that survived beyond the Devonian. Late in the Ordovician, some fishes evolved jaws via modifications of the skeletal arches that supported the gills (FIGURE 23.40). Those fishes and their descendants are referred to as gnathostomes (Greek, “jaw mouths”). Jaws greatly improved feeding efficiency, as an animal with jaws can grasp, subdue, and swallow large prey. Jawed fishes rapidly diversified during the Devonian period, eventually replacing the jawless fishes in dominance of the seas.
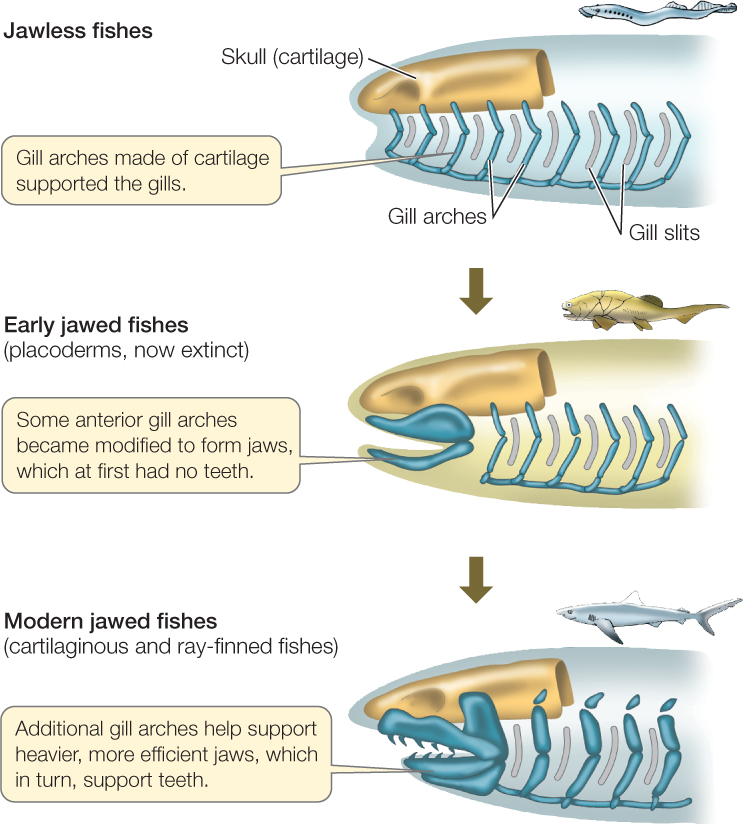
The earliest jaws were simple, but the evolution of teeth made feeding even more efficient. In predators, teeth function crucially both in grasping and in breaking up prey. In both predators and herbivores, teeth enable an animal to chew both soft and hard body parts of their food. Chewing also aids chemical digestion and improves an animal’s ability to extract nutrients from its food.
Fins and swim bladders improved stability and control over locomotion
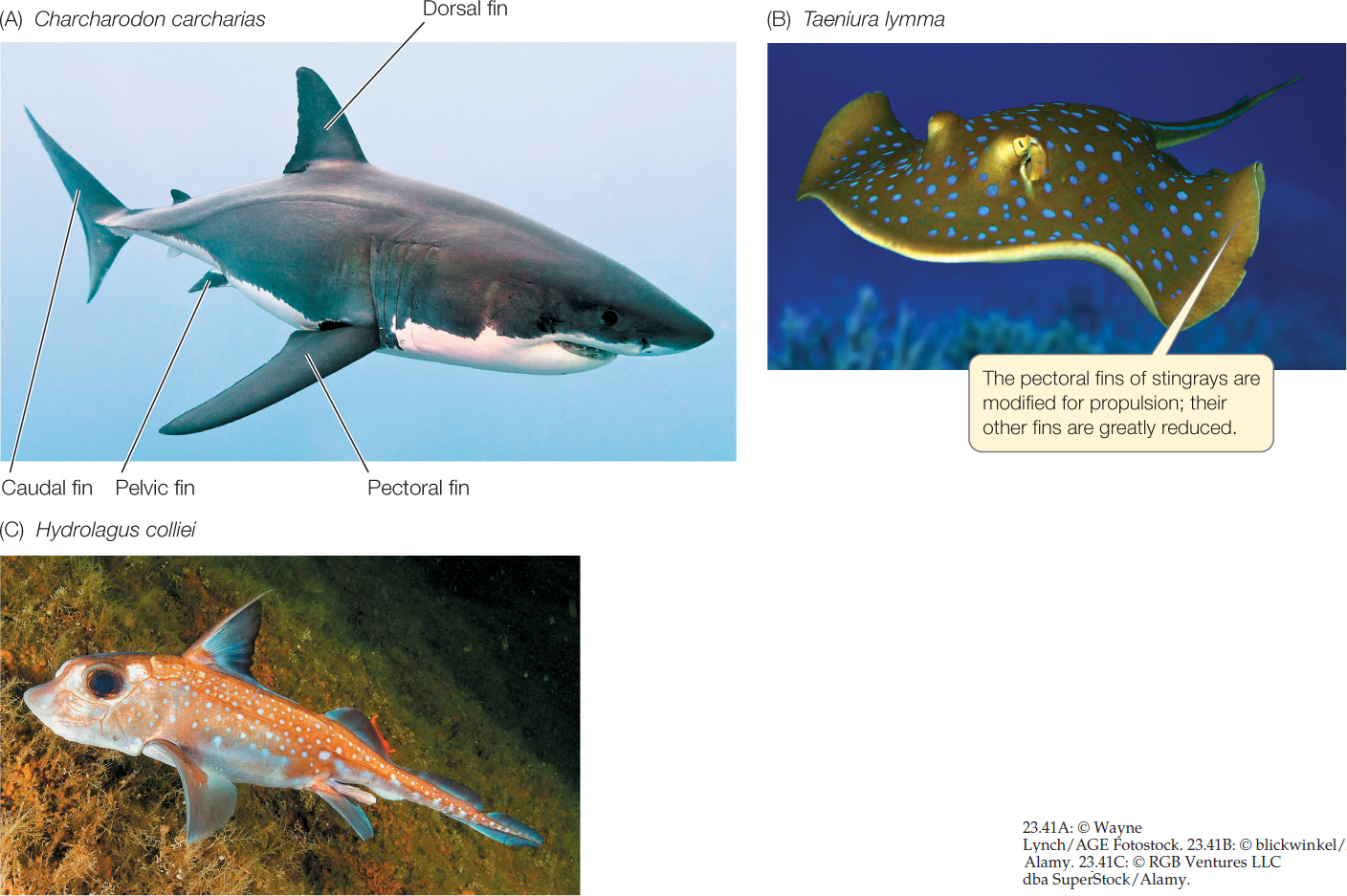
Most jawed fishes have a pair of pectoral fins just behind the gill slits and a pair of pectoral fins anterior to the anus. Paired fins stabilize the position of jawed fishes in water (and in some cases, help propel them). Median dorsal and anal fins also stabilize the fish, or may be used for propulsion in some species. In many fishes, the caudal (tail) fin helps propel the animal and enables it to turn rapidly.
Several groups of gnathostomes became abundant during the Devonian. Among them were the chondrichthyans—sharks, skates, and rays (about 1,000 living species) and chimaeras (40 living species). Like hagfishes and lampreys, these fishes have a skeleton composed entirely of firm but pliable cartilage. Their skin is flexible and leathery, sometimes bearing scales that give it the consistency of sandpaper. Sharks move forward by means of lateral undulations of their body and caudal fin (FIGURE 23.41A). Skates and rays propel themselves by means of vertical undulating movements of their greatly enlarged pectoral fins (FIGURE 23.41B).
504
Most sharks are predators, but some feed by straining plankton from the water. Most skates and rays live on the ocean floor, where they feed on mollusks and other animals buried in the sediments. Nearly all cartilaginous fishes live in the oceans, but a few are estuarine or migrate into lakes and rivers. One group of stingrays is found in river systems of South America. The less familiar chimaeras (FIGURE 23.41C) live in deep-sea or cold waters.
One lineage of aquatic gnathostomes gave rise to the bony vertebrates, which soon split into two main lineages—the ray-finned fishes and the lobe-limbed vertebrates. Bony vertebrates have internal skeletons of calcified, rigid bone rather than flexible cartilage. In early bony vertebrates, gas-filled sacs that extended from the digestive tract supplemented the gas exchange function of the gills by giving the animals access to atmospheric oxygen. These features enabled those fishes to live where oxygen was periodically in short supply, as it often is in freshwater environments. These lunglike sacs evolved into swim bladders (organs of buoyancy), as well as into the lungs of tetrapods. By adjusting the amount of gas in its swim bladder, a fish can control the depth at which it remains suspended in the water while expending very little energy to maintain its position.
The outer body surface of most species of ray-finned fishes is covered with flat, thin, lightweight scales that provide some protection or enhance movement through the water. The gills of ray-finned fishes open into a single chamber covered by a hard flap, called an operculum. Movement of the operculum improves the flow of water over the gills, where gas exchange takes place.
Ray-finned fishes began to radiate during the Mesozoic era and continued to radiate extensively throughout the Tertiary period. Today there are about 32,000 known living species, encompassing a remarkable variety of sizes, shapes, and lifestyles (FIGURE 23.42). The smallest are less than 1 centimeter long as adults; the largest weigh as much as 900 kilograms. Ray-finned fishes exploit nearly all types of aquatic food sources. In the oceans they filter plankton from the water, rasp algae from rocks, eat corals and other soft-bodied colonial animals, dig animals from soft sediments, and prey on virtually all kinds of other fishes. In fresh water they eat plankton, devour insects, eat fruits that fall into the water in flooded forests, and prey on other aquatic vertebrates and, occasionally, terrestrial vertebrates.

CHECKpointCONCEPT23.5
- What are three developmental patterns the earliest deuterostomes had in common?
- What are some of the ways that echinoderms use their tube feet to obtain food?
- What synapomorphies respectively characterize the chordates and the vertebrates?
- How do the hagfishes differ from the lampreys in morphology and life history? Why do some biologists not consider the hagfishes to be vertebrates?
505
In some fishes, the lunglike sacs that gave rise to swim bladders became specialized for another purpose: breathing air. That adaptation set the stage for the vertebrates to move onto the land.