CONCEPT31.3 The Mammalian Breathing System Is Anatomically and Functionally Elaborate
In mammals, air enters the lungs through the oral cavity and the nasal passages, which join together in the pharynx, or throat (FIGURE 31.11A). Below the pharynx, the esophagus, or food tube, directs food to the stomach, and the trachea, or windpipe, leads to the lungs. At the beginning of the trachea is the larynx, or voice box, which houses the vocal cords. The walls of the trachea and many further branches of the airways contain rings of cartilage, which help prevent airway collapse.
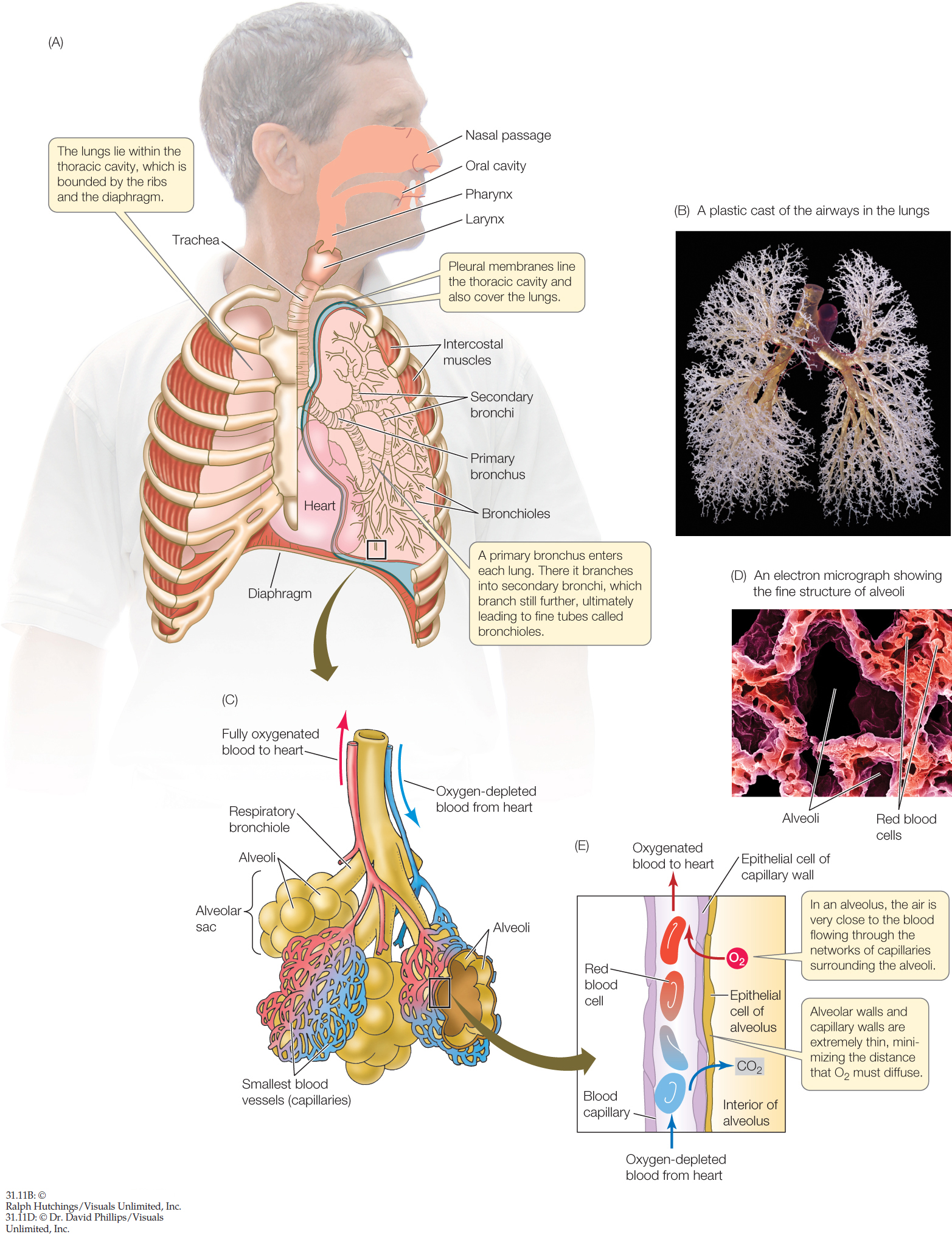
The trachea branches into two primary bronchi, one leading to each lung. Each primary bronchus then branches more than 20 times to generate the treelike structure of progressively smaller airways we discussed earlier (FIGURE 31.11B). At the farthest reaches of the branching airways, each tiny branch ends blindly in an alveolar sac of epithelial tissue (FIGURE 31.11C). The wall of each alveolar sac consists of numerous semispherical pocket-like structures called alveoli (singular alveolus). These alveoli number about 300 million in the two lungs of an adult person, creating an enormous surface area (FIGURE 31.11D) where gas exchange occurs (FIGURE 31.11E).
Most branches of the airway system in a mammal are not involved in the exchange of O2 and CO2 between the air and blood. These branches—including the trachea, primary bronchi, and most of the succeeding branches—are called the conducting airways because their principal function is to conduct flowing air in and out of the lungs during inhalation and exhalation.
Exchange of O2 and CO2 between the air and blood occurs in the respiratory airways. These are the parts of the lungs where the membranes between air and blood are thin and perfused with blood at high rates. The respiratory airways include the last few branches of the branching airway system—termed respiratory bronchioles—and the alveolar sacs, including the alveoli. In healthy human lungs, blood and air are separated by only about 0.5 μm in the alveoli. Bulk air flow—inflow and outflow—takes place in the respiratory bronchioles as a person inhales and exhales. But the air inside the alveoli is motionless. This means that as O2 and CO2 travel to and from the gas exchange membranes, they must diffuse across the central cavities of the alveoli. Moreover, as we already discussed, O2 and CO2 must diffuse across the two thin epithelia that line the alveoli and the surrounding blood capillaries. Diffusion occurs rapidly because the distances to be covered are minute.
655
656
At rest, only a small portion of the lung volume is exchanged
The amount of air that moves in and out of the lungs per breath is called the tidal volume (FIGURE 31.12). When a person is at rest, it is called the resting tidal volume and amounts to about 0.5 liter. The maximal tidal volume—also called vital capacity—is the amount of air that moves in and out per breath when we inhale and exhale as vigorously as possible. It is about 5 liters. We use only about 10 percent of our maximal tidal volume when we breathe at rest.
RESEARCH TOOLS
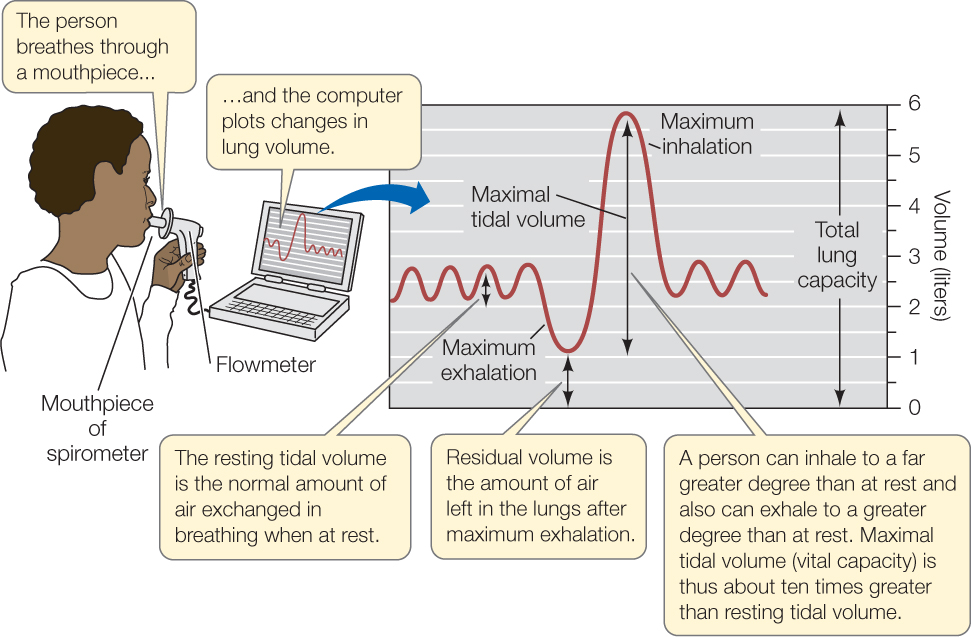
A person’s respiratory minute volume is the total volume of air that he or she inhales and exhales per minute. We calculate the minute volume by multiplying tidal volume (the volume exchanged per breath) by the number of breaths per minute. When people exercise and “breathe harder,” they increase their tidal volume above the resting tidal volume, and they also take more breaths per minute than they do at rest. These two factors can produce a large increase in minute volume. At rest we breathe about 12 times per minute. With a resting tidal volume of about 0.5 liter, our resting minute volume is about 6 liters per minute. Well-conditioned athletes can breathe at least 30 times per minute at a tidal volume of over 3 liters, meaning they can increase their minute volume by a factor of more than 15!
Mammals and other animals with tidally ventilated lungs do not empty their lungs completely when they exhale. Some air is always left behind in the lungs. The amount left after a maximum exhalation is called the residual volume (see Figure 31.12). During normal breathing, whenever a mammal inhales, the fresh air mixes with stale air from the previous breath that was not exhaled. This mixing lowers the O2 partial pressure at the alveolar gas exchange membranes but ordinarily presents no problems.
The lungs are ventilated by expansion and contraction of the thoracic cavity
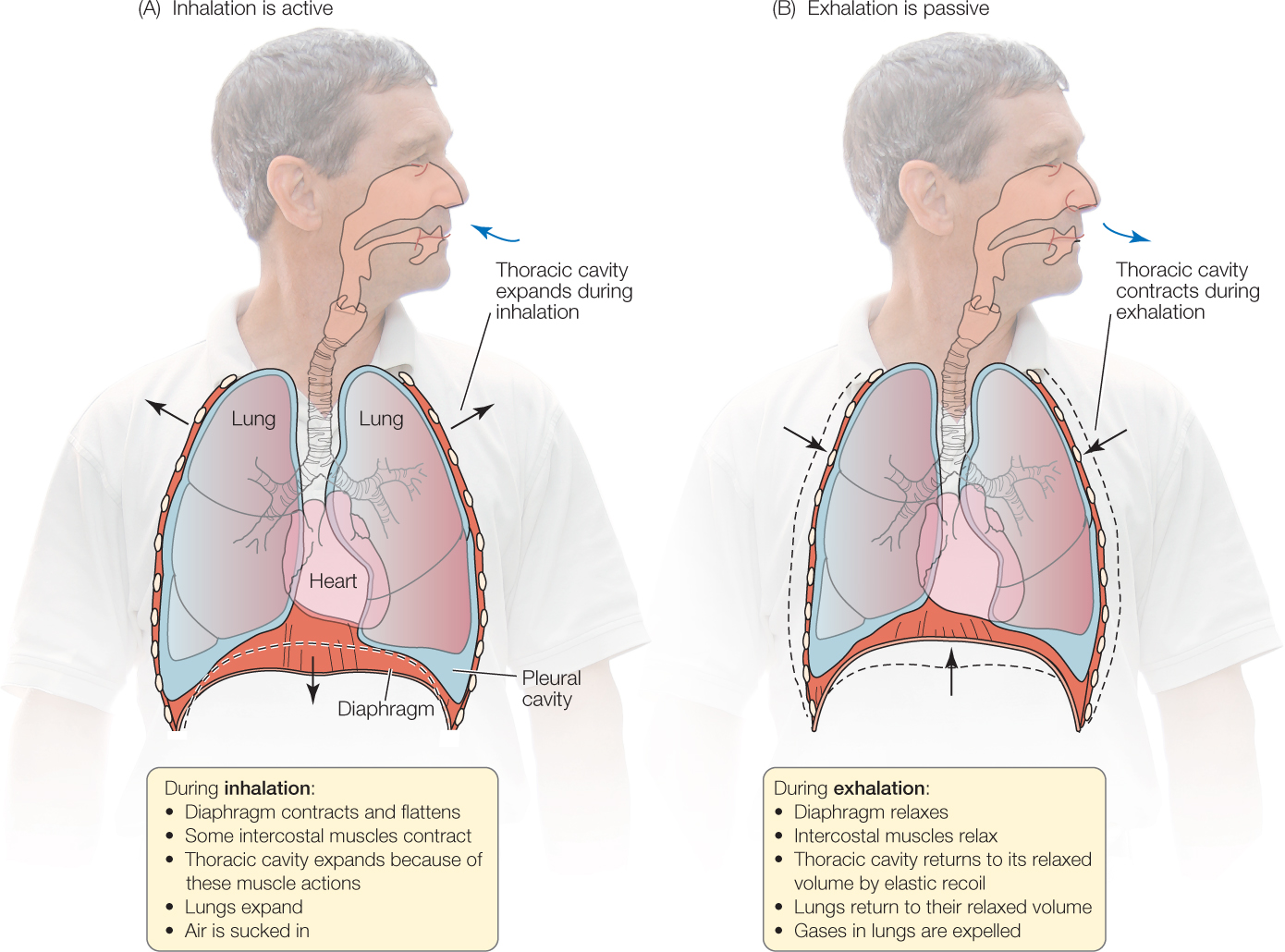
Mammalian lungs are suspended in the thoracic, or chest, cavity, which is bounded on the top by the bones of the shoulders, on the sides by the rib cage, and on the bottom by a sheet of muscle, unique to mammals, called the diaphragm. Any increase in volume of the thoracic cavity pulls on the walls of the lungs, thereby expanding the lungs and causing inflation.
Two muscles or sets of muscles are critical for breathing: the diaphragm and the intercostal (from the Latin costa, “rib”) muscles that run between adjacent ribs (see Figure 31.11A). The diaphragm is dome-shaped at rest, protruding upward from the abdomen into the thoracic cavity. It flattens when it contracts, so it protrudes less into the thoracic cavity, expanding it. There are two sets of intercostal muscles. One set enlarges the thoracic cavity when it contracts, and the other set makes it smaller when it contracts.
In humans at rest, inhalation is active but exhalation is passive (FIGURE 31.13). During inhalation, the diaphragm and certain intercostal muscles contract, expanding the thoracic cavity and lungs, which fill by suction (a lower pressure in the lungs than in the atmosphere). During exhalation at rest, these muscles simply relax, allowing the elastic recoil of the lungs and thoracic cavity to restore the relaxed lung volume. During exercise, additional breathing muscles come into play. Inhalation is more forceful, and muscle contractions are employed to make exhalation faster and more forceful than it is at rest.
The breathing rhythm depends on nervous stimulation of the breathing muscles
All the muscles involved in breathing require nervous stimulation to contract. The nerve impulses originate in the brain. These facts explain why a broken neck can kill a person rapidly. If the nerves carrying the breathing impulses from the brain to the breathing muscles are severed, the muscles stop contracting. Without artificial respiration, the person suffocates.
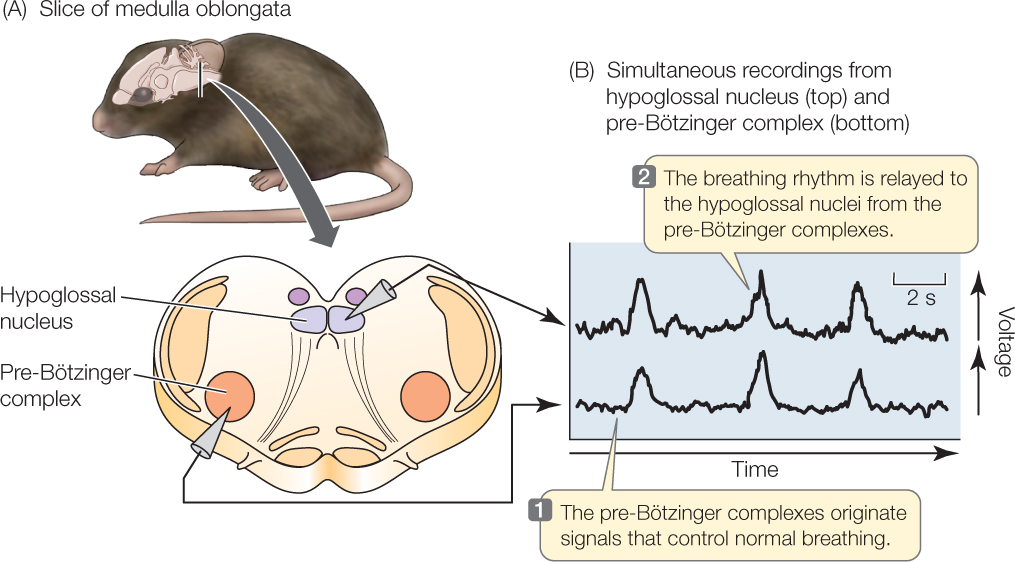
Physiologists have long sought the places in the brain where the breathing rhythm originates. Investigators recently identified these rhythm-generating (rhythmogenic) centers by studying thin slices of brain tissue taken from nestling rodents. Although these slices are cut off from all their normal connections with other parts of the nervous system, they remain functional for some time. Investigators have shown that two neural complexes in the medulla oblongata of the brain (a part of the brainstem) generate a breathing rhythm under these circumstances (FIGURE 31.14). These rhythm-generating centers, called the pre-Bötzinger complexes, are believed to be the beginning points for the nerve impulses that activate the muscles we use in breathing.
657
658
Breathing is under negative-feedback control by CO2
You might expect that a mammal’s breathing would be regulated by the blood’s O2 content. In fish, blood O2 is indeed the primary feedback stimulus for gill ventilation. However, mammals are relatively insensitive to falling levels of O2 in arterial blood. Instead they are very sensitive to changes in blood CO2.
To fully understand control of breathing by CO2, it is important to recognize that CO2 dissolved in the blood reacts with blood H2O. This reaction forms carbonic acid (H2CO−), which dissociates almost completely into H+ ions and bicarbonate ions (HCO3−).
659
CO2 + H2O ⇋ H2CO3 ⇋ + HCO3−
What happens when the cells of your body increase their metabolism and therefore add CO2 to the blood at an increased rate? There are two immediate consequences. First, the partial pressure of CO2 in the blood rises. Second, the concentration of H+ in the blood also rises, meaning the blood becomes more acidic and its pH falls. Chemosensitive (chemical-sensing) neural centers located on the ventral surface of the medulla oblongata in the brain (see Figure 31.14) independently monitor the blood CO2 partial pressure and H+ concentration. These centers detect the increases in CO2 partial pressure and H+ concentration. This information is then used to control breathing in a negative-feedback manner. Breathing is stimulated so that the respiratory minute volume increases. This increased ventilation increases exhalation of CO2, helping the body get rid of CO2 at an accelerated rate when the cells have increased their rate of CO2 production. The increased ventilation also tends to return both the blood CO2 partial pressure and H+ concentration back toward their values that existed before CO2 production increased.
What if, under different circumstances, your cells decrease the rate at which they add CO2 to the blood? In this case, the blood CO2 partial pressure and H+ concentration initially tend to fall. The chemosensitive centers detect these changes, leading again to negative-feedback control of breathing. In this instance, breathing is inhibited, lowering the rate of CO2 exhalation and causing the blood CO2 partial pressure and H+ concentration to rise.
These controls serve to adjust the respiratory minute volume so that the rate of CO2 exhalation approximately matches the rate at which the cells of the body produce CO2. The controls also stabilize the blood CO2 partial pressure and H+ concentration, by tending to bring them down if they start to rise and tending to bring them up if they start to fall.
In the experiment in FIGURE 31.15, dogs ran on a treadmill at a constant speed. The slope of the treadmill could be tilted up at various angles, allowing the researchers to vary the dogs’ metabolic workload and thus their rates of CO2 production. Negative-feedback systems rarely lead to complete stability. In this case, the CO2 partial pressure in a dog’s arterial blood tended to vary a little (it would have varied much more without negative feedback). The results demonstrate that a dog’s respiratory minute volume is closely correlated with its blood CO2 partial pressure. This evidence supports the idea that breathing is stimulated when the blood CO2 partial pressure increases.
Investigation
HYPOTHESIS
Arterial CO2 partial pressure is a feedback stimulus controlling ventilation.
- Dogs are trained to run on a treadmill.
- The dogs are equipped with instruments that measure respiratory minute volume and with arterial catheters that enable sampling of blood.
- As a dog runs at a constant speed, the slope of the treadmill is changed to modify the metabolic workload.
- Minute volume is plotted as a function of arterial CO2 partial pressure.
When the workload is altered by slowly changing the slope of the treadmill (no change in speed), the respiratory minute volume is a function of arterial CO2 partial pressure.
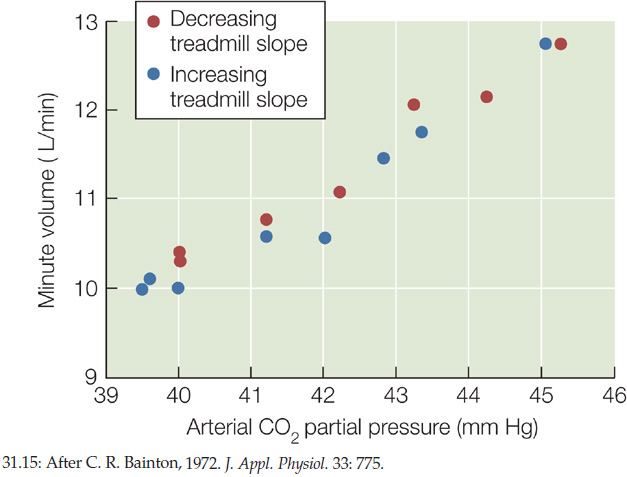
CONCLUSION
The data support the hypothesis that arterial CO2 partial pressure is a metabolic feedback signal controlling ventilation in response to changes in workload.
ANALYZE THE DATA
Additional experiments were done in which the speed of the treadmill was changed instead of the slope to modify the metabolic workload. Again the respiratory minute volume (L/min) and arterial CO2 partial pressure (PCO2, in units of mm Hg) were measured. The average values when the dogs were running at 3 mph were CO2 partial pressure = 39.0 mm Hg and L/min = 9. The data for the first seven breaths after the treadmill speed was increased to 6 mph are shown in the table. After the dogs had run at 6 mph for several minutes, the values averaged CO2 partial pressure = 41.0 mm Hg and L/min = 15.0. Plot these data and use the information to answer the questions.
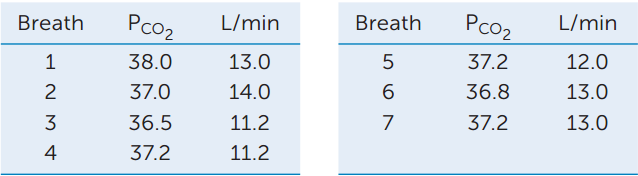
- Do these data support the hypothesis that CO2 partial pressure is the feedback signal controlling ventilation? Why or why not?
- How do you explain the average values after the dogs had been running at the higher speed for a few minutes?
- Relate these results to those obtained in the experiment in which the slope of the treadmill was varied. Explain the differences between the results.
aC. R. Bainton. 1972. Journal of Applied Physiology 33: 778–787.
Breathing is also under control of factors in addition to CO2
You may have noticed that when you start running, your breathing rate increases almost immediately—far sooner than your blood CO2 partial pressure would be expected to change. This everyday observation suggests that the simple act of moving muscles and joints may serve to accelerate ventilation during exercise. Investigators studied this possibility in the experiments on dogs we’ve been discussing (see Analyze the Data in Figure 31.15). For this purpose the dogs ran on a horizontal treadmill, and investigators altered the dogs’ intensity of work by varying how fast they ran. When the dogs worked harder, respiratory minute volume sometimes increased to a greater extent than CO2 partial pressure by itself would explain. According to the results of this study and other studies, information from receptors that detect motion in muscles and joints is used during exercise to help control the intensity of ventilation.
Blood O2 partial pressure is also sometimes used in controlling the intensity of ventilation. O2 partial pressure is not as influential as CO2 partial pressure, and minor fluctuations in the O2 partial pressure have little effect on ventilation. However, a large drop in blood O2 partial pressure—such as might occur at high elevation—strongly stimulates ventilation.
In mammals, two sets of oxygen-sensing chemosensitive bodies detect the blood O2 partial pressure. They are called peripheral chemoreceptors because they are not in the brain. They are positioned along the large arteries leaving the heart (see Figure 32.6). One set (by far the most important set in humans) is a pair of carotid bodies found along the carotid arteries in the neck. The second set consists of aortic bodies associated with the aorta. These bodies detect blood O2 partial pressure and send information on it by way of nerves to the breathing control centers in the medulla oblongata.
CHECKpointCONCEPT31.3
- The alveoli become filled with tissue fluid in several types of diseases, such as heart weakening that causes pulmonary edema. This condition is a health emergency. Why?
- In a healthy individual, how would the respiratory minute volume change in response to a decrease in blood pH?
- How could a neck injury that leaves the trachea and other air passageways intact result in an inability to breathe?
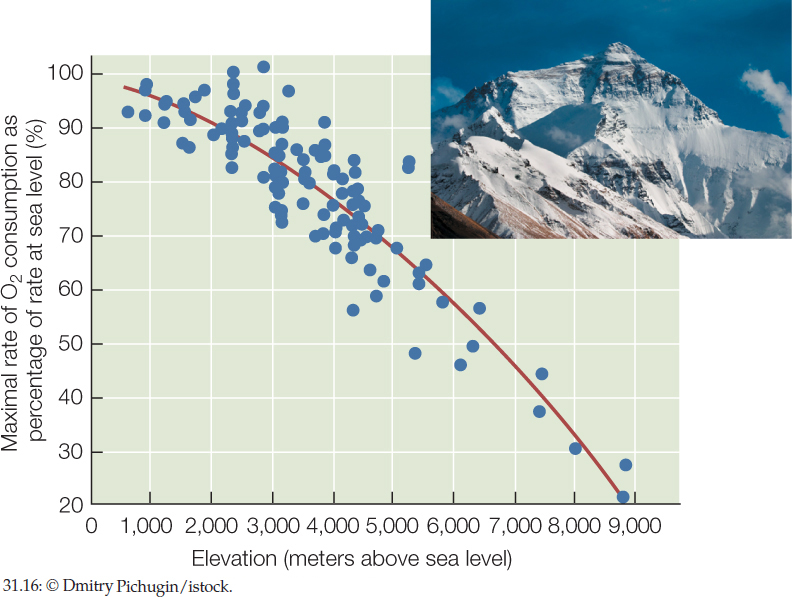
Why is climbing uphill at 1.7 meters per minute (100 meters/hour) the most intense work that is possible for a human near Everest’s summit?
ANSWER Any particular type of sustained, physical work demands a certain amount of sustained metabolic effort and thus a certain, fixed rate of O2 consumption (regardless of elevation). Consequently a person’s maximal rate of O2 consumption determines the maximal sustained work intensity of which he or she is capable. A person’s maximal rate of O2 consumption decreases with elevation because O2 becomes more dilute in the atmosphere as elevation increases (FIGURE 31.16). For this reason, maximal work intensity decreases with elevation. At the top of Mount Everest, hardly any work is possible. The maximal intensity of work is a slow walk.
660