CONCEPT32.3 A Beating Heart Propels the Blood
A heart is a discrete, localized pumping structure. Some animals with a circulatory system lack a heart. In earthworms and other annelid worms, for example, the blood is propelled entirely by peristaltic waves of contraction in muscular blood vessels. Some animals, by contrast, have more than one heart. Crayfish and lobsters, for instance, have a small accessory heart that helps perfuse their brain, in addition to their large, principal heart.
The muscle tissue of a heart is termed the myocardium (myo, “muscle”; cardi, “heart”). The cells of the myocardium often have distinctive properties, compared with skeletal muscle cells or smooth muscle cells. They are thus distinguished as cardiac muscle cells (see Concept 33.4).
The volume of blood pumped per beat by a heart is called the heart’s stroke volume. A heart’s cardiac output is the volume of blood the heart pumps per minute. We calculate cardiac output by multiplying stroke volume by the number of beats per minute, the heart rate. For example, when you are at rest, your heart has a stroke volume of about 70 milliliters per beat (volume pumped into the systemic circuit) and beats about 70 times per minute. Your resting cardiac output is thus about 5 liters per minute (70 ml/beat × 70 beats/min = 4,900 ml/min, or about 5 L/min).
During exercise, we increase both stroke volume and heart rate. An average young person can increase cardiac output about fourfold. Even at rest, however, the amazing performance of the heart is evident. At rest the heart pumps about 300 liters each hour, corresponding to 2.6 million liters per year and almost 200 million liters in an average lifetime.
Vertebrate hearts are myogenic and multi-chambered
Hearts are classified based on the answers to two questions: how is each beat initiated, and how many chambers are present?
Vertebrate hearts do not require nervous stimulation to beat. Instead, each beat is initiated within the myocardium in modified muscle cells, and the muscle of a vertebrate heart beats on its own, even when all connections to other tissues of the body are severed. Vertebrate hearts are classed as myogenic, meaning “beginning in muscle,” to recognize that heartbeats originate in the muscle tissue. Mollusks, too, have myogenic hearts. Crustaceans do not, however, as we will see later.
Vertebrate hearts are also classed as multi-chambered because they consist of two or more distinct chambers. Fish hearts have two muscular chambers: an atrium (plural atria)—which is a thin-walled chamber through which blood passes on its way to the principal pumping chamber—and a thick-walled, highly muscular ventricle. Amphibian hearts have three chambers (two atria that empty into one partially divided ventricle), and the hearts of mammals and birds have four chambers. One-way valves are present where one heart chamber opens into another, ensuring than blood flows through the chambers in the correct order.
Let’s take a closer look at the mammalian heart (FIGURE 32.6). It consists of four chambers: two highly muscular ventricular chambers, which contract together, and two less-muscular atria, which also contract together. O2-depleted blood returning to the heart from the systemic tissues enters the right atrium and then flows through a one-way valve into the right ventricle. When the ventricles contract, the O2-depleted blood is pumped into the pulmonary arteries leading to the lungs. O2-rich blood delivered to the heart from the lungs enters the left atrium and then passes through a one-way valve into the left ventricle. When the ventricles contract, this O2-rich blood is pumped into a massive artery, the aorta, from which it flows through branching arteries to the systemic tissues.
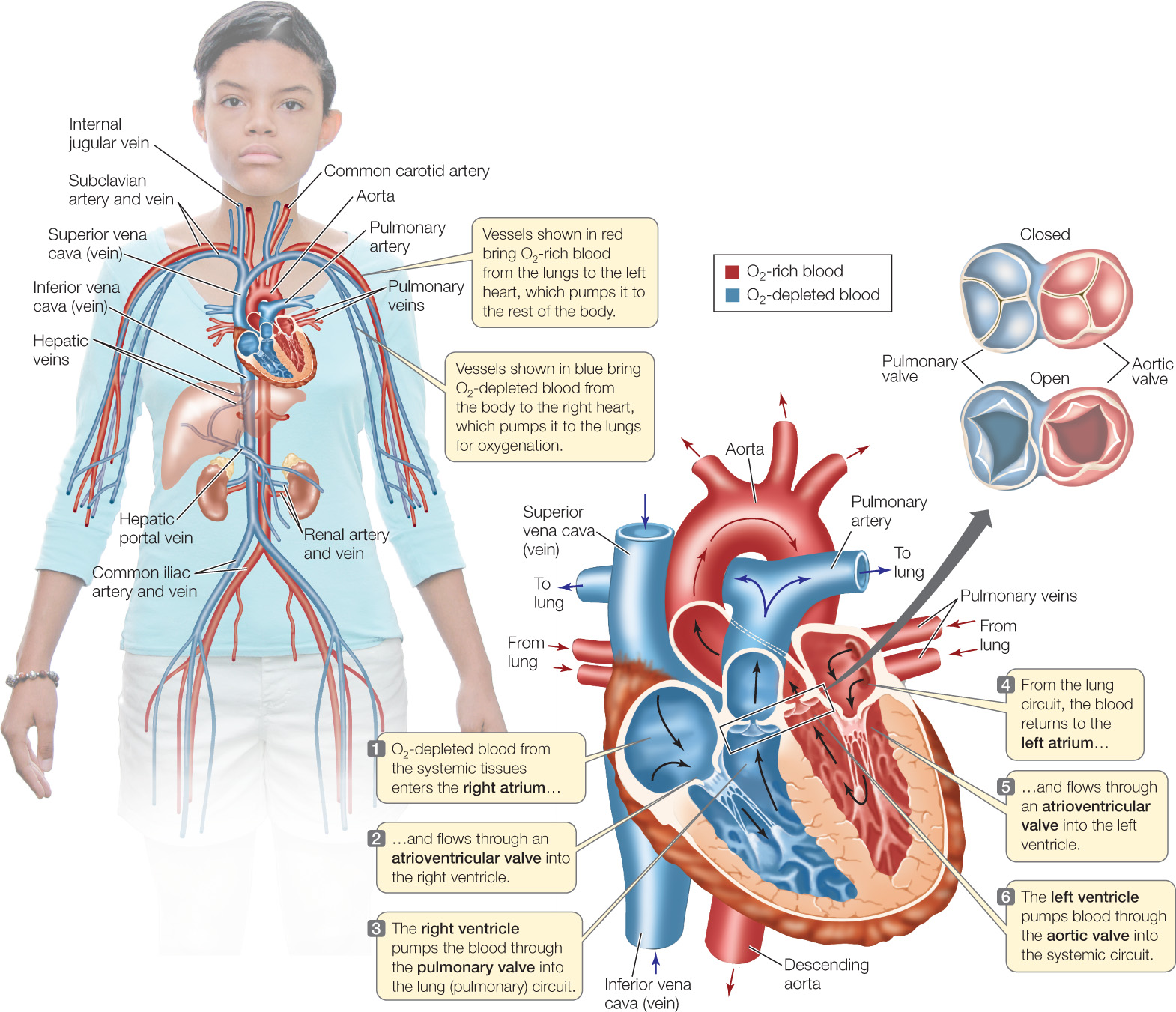
The cycle of contraction and relaxation of the heart is called the cardiac cycle. This cycle is divided into two phases: systole (pronounced sís-toll-ee), when the ventricles contract, and diastole (die-ás-toll-ee), when the ventricles relax (FIGURE 32.7).
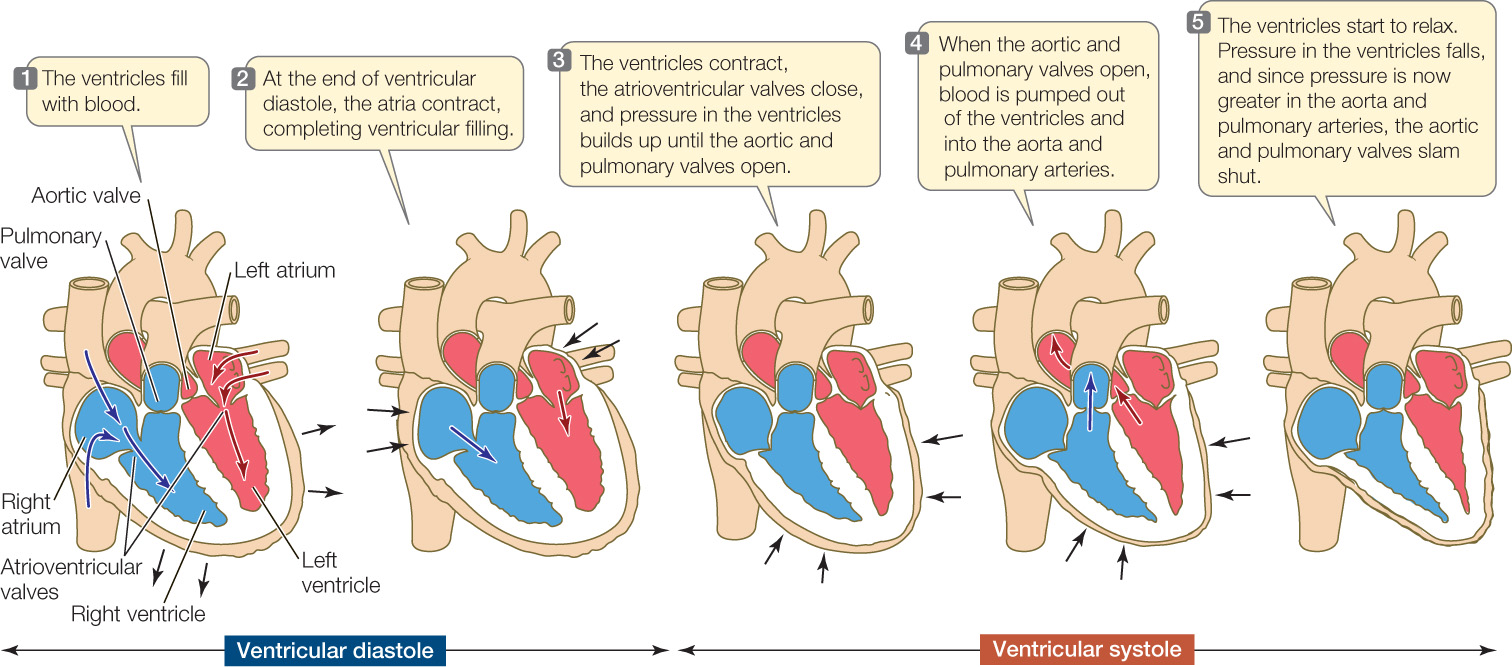
The ventricles pressurize the blood inside them when they contract, and this positive pressure—developed by contraction of the ventricular myocardium—is the principal force that drives blood through the blood vessels. The positive pressure developed by the right ventricle forces blood to flow to the lungs and back to the heart. The positive pressure developed by the left ventricle forces blood to flow to all the systemic tissues and back. The left ventricle is more muscular than the right (see Figure 32.6) and develops higher pressures, which help ensure flow through the larger systemic circuit.
668
The blood pressure at any point in the circulatory system is the extent to which the pressure in the blood exceeds the pressure in the environment of the animal. As you might imagine, the blood pressure in the aorta rises and falls during the cardiac cycle, even though the aortic walls are elastic and therefore tend to dampen pressure changes. In healthy young people, the aortic pressure during systole is about 120 mm Hg (millimeters of mercury, referring to the height of a vertical column of mercury that the pressure can support). The aortic pressure during diastole is about 80 mm Hg. These pressures undergo little change as blood flows through major arteries and can therefore be measured in the arm, as is usually done. They are typically quoted as a ratio: “120 over 80.” Hypertension (“high blood pressure”) is a disease in which the pressures are significantly higher than this norm.
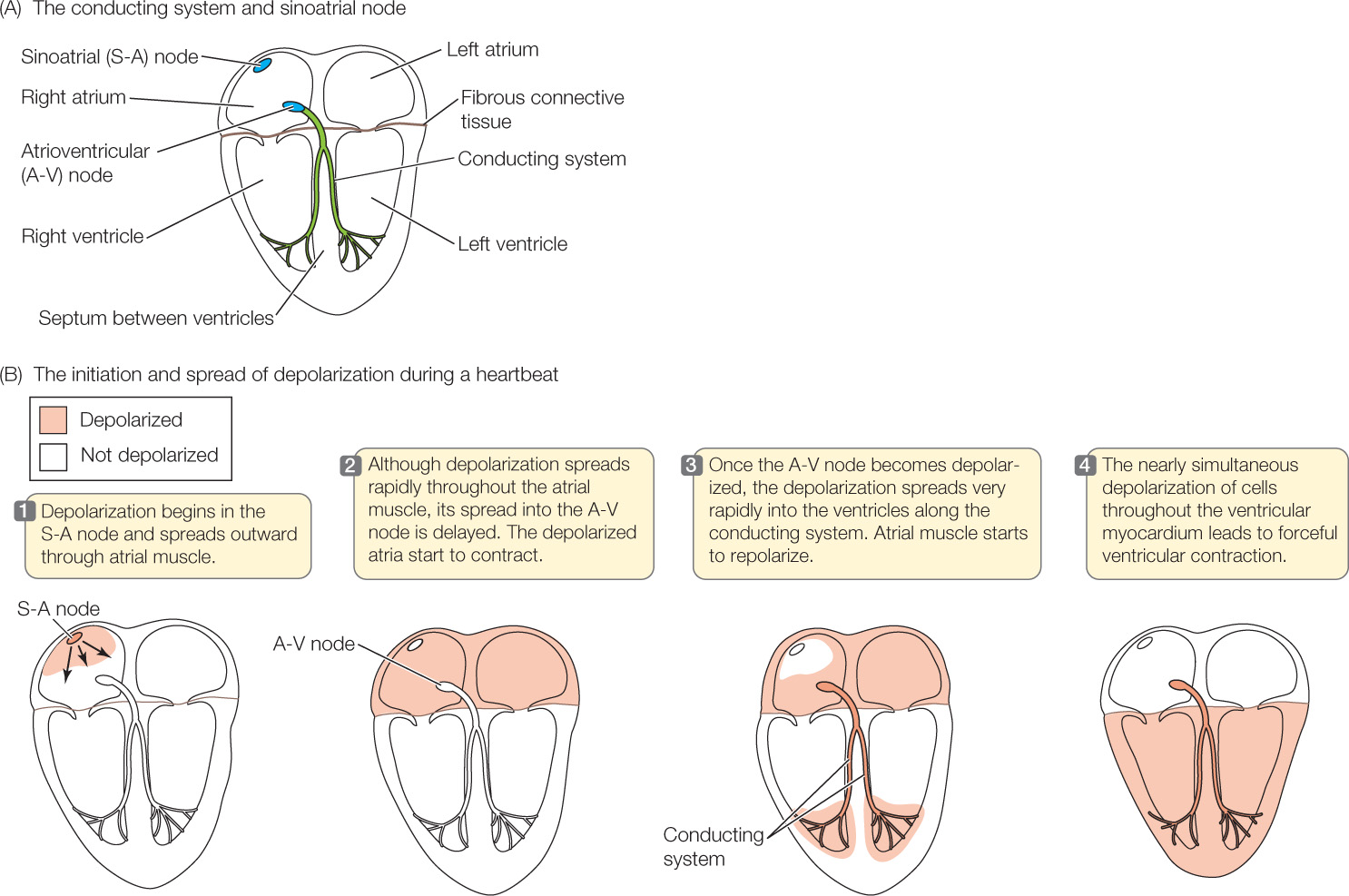
Each heartbeat is initiated by pacemaker cells, which are modified muscle cells found in a localized part of the right atrial wall, the sinoatrial (S-A) node (Figure 32.8A). Cardiac muscle cells in the vertebrate myocardium are electrically coupled, meaning that when a particular cell undergoes electrical depolarization (a reversal of the electric charge difference across the cell membrane) and consequently contracts, it initiates electrical depolarization and contraction in the cells next to it. Accordingly, when electrical depolarization and contraction are initiated at any one point in a mass of heart muscle, they spread rapidly throughout the muscle by moving from cell to cell. This process ensures that all the cells within a large mass of muscle depolarize and contract almost simultaneously. After the pacemaker cells in the S-A node initiate a wave of depolarization and contraction, the depolarization and contraction rapidly spread throughout the atria (Figure 32.8B, steps  and
and  ). Depolarization cannot spread directly into the ventricles, however, because a sheet of fibrous connective tissue lies between the atrial and ventricular muscle masses.
). Depolarization cannot spread directly into the ventricles, however, because a sheet of fibrous connective tissue lies between the atrial and ventricular muscle masses.
669
670
A beating heart propels the blood
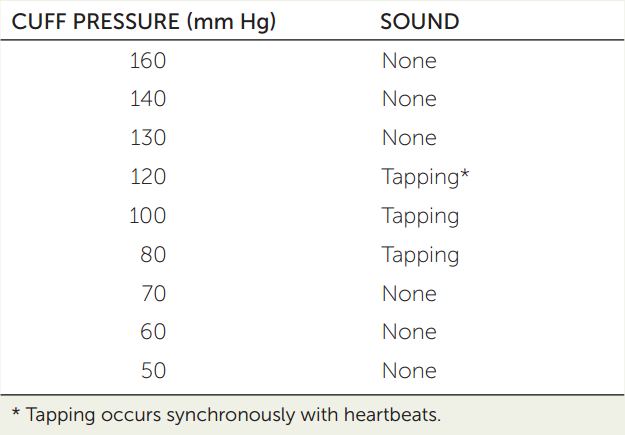
A physician or nurse measures your blood pressure with (1) a stethoscope and (2) an inflatable pressure cuff and a pressure gauge, together called a sphygmomanometer. Typically the pressure cuff is placed around your upper arm, and the stethoscope is used to listen to the sounds of blood flowing through the artery at the crook of your elbow. When the cuff is inflated to a high enough pressure, only a background rumble is heard in the stethoscope. Then the physician or nurse slowly lowers the pressure in the cuff. Suppose the observations shown at the right are obtained.
- Why is there no sound at the high cuff pressure, and why does the tapping start as the pressure is lowered?
- Why does the tapping sound disappear after several steps of lowering the cuff pressure?
- What pressure reading is closest to the systolic pressure? To the diastolic pressure?
LINK
Electrical depolarization in muscle cells is described in Concept 33.1; Concept 33.4 provides more details about the special characteristics of cardiac muscle
How, then, do electrical depolarization and contraction move from the atria to the ventricles? A set of modified muscle cells called the atrioventricular (A-V) node is situated near the junction of the atria and the ventricles (see Figure 32.8A). Electrical depolarization of the atria causes depolarization in this node after a slight delay. This delay is important because it ensures that the ventricles contract after the atria. Once depolarized, the cells of the A-V node relay the electrical depolarization into a system of modified muscle cells called the conducting system (see Figure 32.8A), in which the depolarization travels very rapidly from cell to cell, sweeping at high speed into the right and left ventricles (see Figure 32.8B, step  ). As soon as muscle cells in the ventricles start to depolarize and contract, the wave of depolarization and contraction spreads rapidly from cell to cell within the ventricles (see Figure 32.8B, step
). As soon as muscle cells in the ventricles start to depolarize and contract, the wave of depolarization and contraction spreads rapidly from cell to cell within the ventricles (see Figure 32.8B, step  ). The result is a strong, coordinated ventricular contraction.
). The result is a strong, coordinated ventricular contraction.
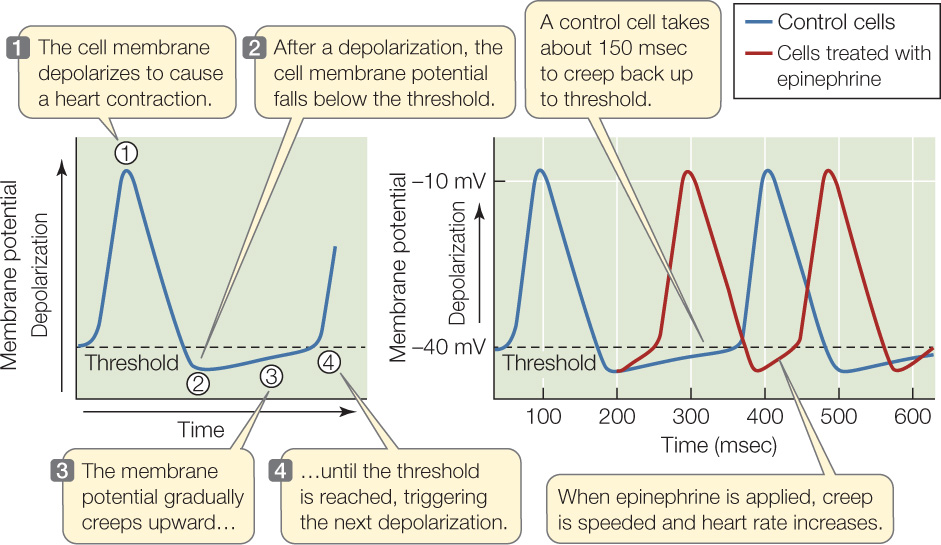
Nerves to the heart and blood-borne hormones can strongly modify and control the beating of the heart. That is, even though the heart beats on its own, it is subject to controlling influences—termed extrinsic controls—exerted by the nervous and endocrine systems. We’ve all felt our heart pound after our adrenal glands secrete a rush of epinephrine and norepinephrine (adrenaline and noradrenaline) in response to fright or stress, and this is the most common way that we become aware of extrinsic controls. Both the pacemaker cells in the S-A node and the ordinary muscle cells of the myocardium are highly innervated by the autonomic nervous system. As experiments along the lines of the Investigation in FIGURE 32.9 show, the autonomic nervous system influences the S-A node to increase or decrease the heart rate. It also influences the ordinary myocardial cells to increase or decrease their force and speed of contraction.
Investigation
HYPOTHESIS
The ANS neurotransmitter epinephrine influences the membrane potentials of pacemaker cells in ways that alter heart rate.
- Culture living S-A node tissue from an experimental animal in a dish. Insert an intracellular recording electrode into pacemaker cells.
- Measure the membrane potential of pacemaker cells during resting heartbeats (the control) and after application of epinephrine.
CONCLUSION
The ANS neurotransmitter epinephrine influences heart rate by altering the membrane potentials of pacemaker cells.
aH. Brown et al. 1979. The Journal of Experimental Biology 81: 175–204.
LINK
The autonomic nervous system controls many involuntary processes, as discussed in Concept 34.5; Concept 35.2 describes the numerous effects of epinephrine and norepinephrine
The myocardium must receive O₂
The cells of the heart muscle in all animals must have a way to obtain O2. We know the most about this process in vertebrates.
In many types of fish, the myocardial cells get their O2 directly from the blood flowing through the heart chambers. However, this way of getting O2 can not meet cellular O2 needs in hard-working, high-performance hearts, explaining the evolution of a coronary circulatory system.
A coronary circulatory system consists of arteries, capillaries, and veins inside (or closely associated with) the myocardium. In animals with a coronary circulation, the heart pumps O2-rich blood into its own coronary arteries. This blood then flows through the coronary arteries to the coronary capillary beds in the myocardium, providing O2 to all the cardiac muscle cells. Mammals and birds depend entirely on a coronary circulation for their cardiac muscle cells to receive O2. Fish with high-performance hearts, such as tuna and salmon, depend in part on a coronary circulatory system.
Coronary artery disease is a common, life-threatening problem for people in today’s world. Blockage of a coronary artery, by fatty deposits or clots, deprives some of the cardiac muscle cells of adequate O2. When blockage becomes complete, a heart attack can occur, and the cells in the affected part of the myocardium can die.
671
An electrocardiogram records the electrical activity of the heart
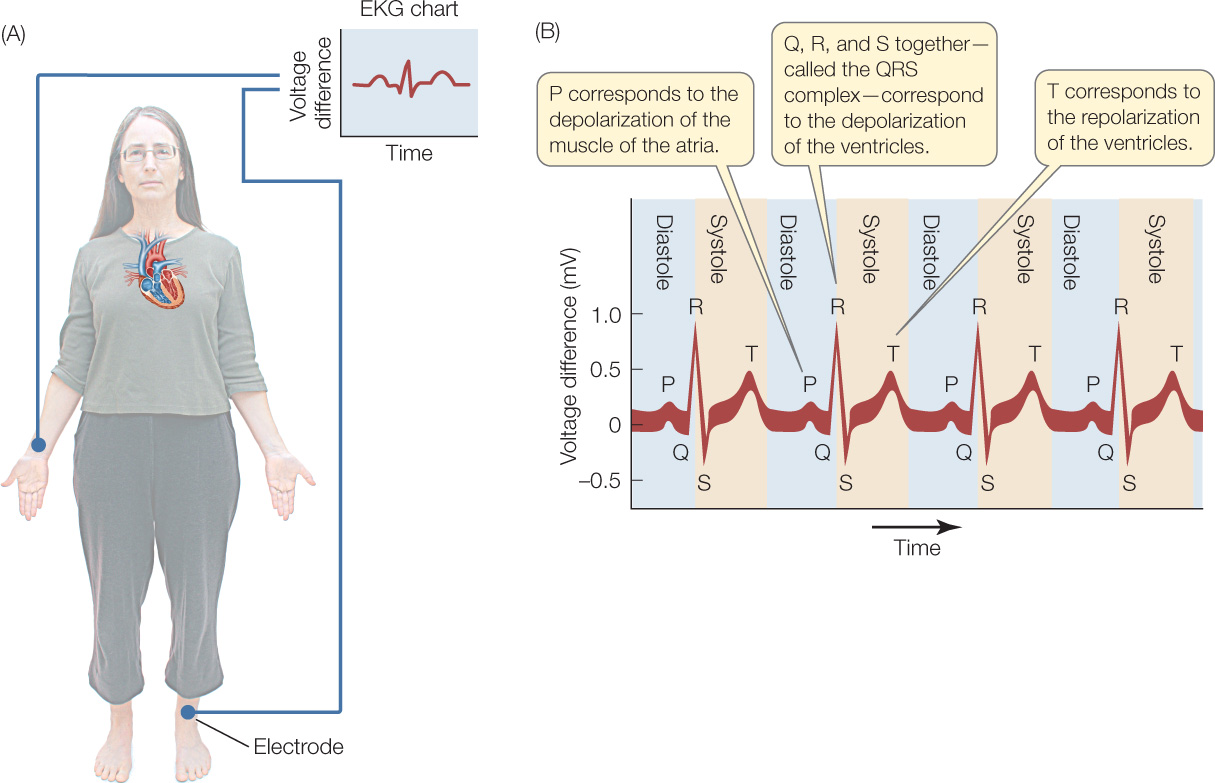
Great numbers of muscle cells depolarize together each time a heart contracts (see Figure 32.8B). Because the cells undergo electrical depolarization simultaneously, their individual voltage signals add together to produce a large voltage signal. This voltage signal is so large, in fact, that it often can be readily detected on the surface of the body! An electrocardiogram (ECG or EKG) is simply a record of heart-related voltage differences on the surface of the body over time. EKGs can be recorded from all sorts of animals, even lizards or octopuses. They are an important tool for diagnosing heart problems in people and pets.
A simple EKG can be recorded using just two electrodes, such as one on the right arm and one on the left leg (FIGURE 32.10). For medical diagnoses, however, recordings are made from many pairs of electrodes, with each pair providing slightly different information.
Crustacean hearts are neurogenic and single-chambered
The hearts of crayfish, lobsters, crabs, and their relatives differ from those of vertebrates in several ways. Study of crustacean hearts thus helps us appreciate that evolution has produced diverse types of circulatory systems in animals.
The muscle cells of crustacean hearts require nervous stimulation to beat. Crustacean hearts are thus neurogenic, meaning that beating “begins in neural tissue.” Some scorpions and spiders also have neurogenic hearts.
A ganglion—a cluster of multiple nerve cells—is associated with the dorsal wall of the single heart chamber in a crustacean heart (Figure 32.11A). One of the nerve cells in this cardiac ganglion acts as a pacemaker cell, rhythmically initiating nerve impulses. These impulses are then relayed by nerve cells to every muscle cell in the heart. The muscle cells in a crustacean heart contract simultaneously during a heartbeat because nerve cells stimulate all of them simultaneously—a very different mechanism than in vertebrate hearts. If the cardiac ganglion and the heart muscle are dissected apart, the ganglion continues to produce rhythmic nerve impulses, but the muscle cells stop contracting.
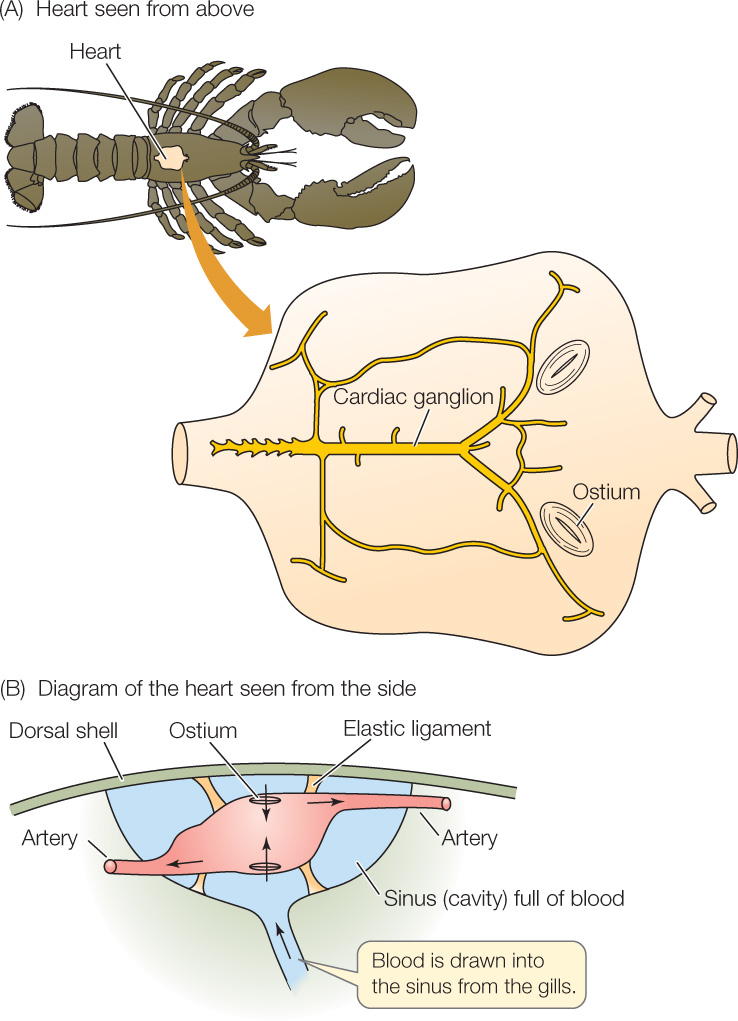
Both positive pressure and suction are crucial for heart function in crustaceans. The single-chambered heart is suspended by elastic ligaments in a blood-filled cavity (Figure 32.11B). All the vessels attached to the heart are arteries and carry blood away from the heart (see Figure 32.4). Three pairs of slits, called ostia (singular ostium), penetrate the heart wall and act as one-way valves that allow blood to flow into but not out of the heart. When the heart contracts, the elastic ligaments are stretched like rubber bands, and the blood inside the heart is pressurized, forcing the blood to flow out into the arteries. Then, when the heart relaxes, the stretched elastic ligaments shorten, pulling the walls of the heart chamber outward and tending to cause suction inside the heart. This suction pulls blood into the heart chamber through the ostia, filling the heart in preparation for the next contraction.
672
CHECKpointCONCEPT32.3
- What is a coronary artery?
- In the human heart, why do the ventricles contract after the atria, even though cardiac muscle cells directly activate neighboring cells?
- What results would you observe if you obtained a living crayfish heart and separated the cardiac ganglion from the heart muscle and then studied the two parts? What conclusions would you draw from the results?
Now that we have discussed circulatory plans and hearts, we will complete our examination of the circulatory system by focusing on the blood vessels.
673