CONCEPT36.3 Aquatic Animals Display a Wide Diversity of Relationships to Their Environment
A key question to ask regarding any water-breathing aquatic animal is, What is the animal’s body fluid composition compared with the composition of the water in which it lives? The simplest way to address this question is to focus on the total solute concentration—the osmotic pressure:
- Some animals have body fluids that have the same osmotic pressure as the water in which they live. These animals are said to be isosmotic (iso, “equal”) to their environment.
- Other animals have body fluids that have a higher osmotic pressure than the environmental water. These animals are termed hyperosmotic, (hyper, “higher”) to their environment.
- Still other animals have body fluids that have a lower osmotic pressure than the environmental water. They are described as hyposmotic (hypo, “lower”) to their environment.
We quantify osmotic pressure in terms of osmolarity. Recall that each separate dissolved entity in a solution—whether it is a glucose molecule, protein molecule, or Na+ ion—contributes approximately equally to osmotic pressure. A solution is defined to be 1 osmolar—symbolized 1 Osm—if it has 6 × 1023 dissolved entities per liter (6 × 1023 is Avogadro’s number). A solution that is 1 osmolar is 1,000 milliosmolar, symbolized mOsm.
Most invertebrates in the ocean are isosmotic with seawater
The simplest and energetically cheapest way for an animal to live in the ocean is for its body fluids to be the same in their total solute concentration as seawater. Animal life first appeared in the ocean, and we think the earliest animals were simply isosmotic with the seawater. Today nearly all invertebrates in the ocean—including lobsters, squid, corals, and sea stars—are isosmotic with seawater. Seawater has an osmotic pressure of almost exactly 1 Osm. The blood and other body fluids of most marine invertebrates thus have an osmotic pressure of about 1 Osm.
Ocean bony fish are strongly hyposmotic to seawater
There are more than 20,000 species of fish with bony skeletons in the ocean. Their body fluids are far more dilute, in terms of both osmotic pressure and the concentrations of major ions, than the seawater in which they swim. The osmotic pressure of their blood plasma and other body fluids is 0.3–0.5 Osm (300–500 mOsm), whereas that of seawater is 1 Osm. The fish maintain this difference by use of regulatory mechanisms and thus are classed as hyposmotic regulators.
Recall from our opening story about salmon that the gills, mouth membranes, and some of the other body surfaces of fish are permeable to water and salts. Because of this permeability and their dilute body fluids, ocean fish face two relentless, never-ending problems:
- They tend to lose water by osmosis from their dilute body fluids into the surrounding, more concentrated seawater (FIGURE 36.5A). Their steady loss of water tends to dehydrate them (living in the ocean is like living in a desert for these fish).
- They also tend to gain ions, such as Na+ and Cl−, by diffusion from the seawater into their more dilute body fluids. Their steady losses of water and gains of ions tend to make their blood and other body fluids become too concentrated.
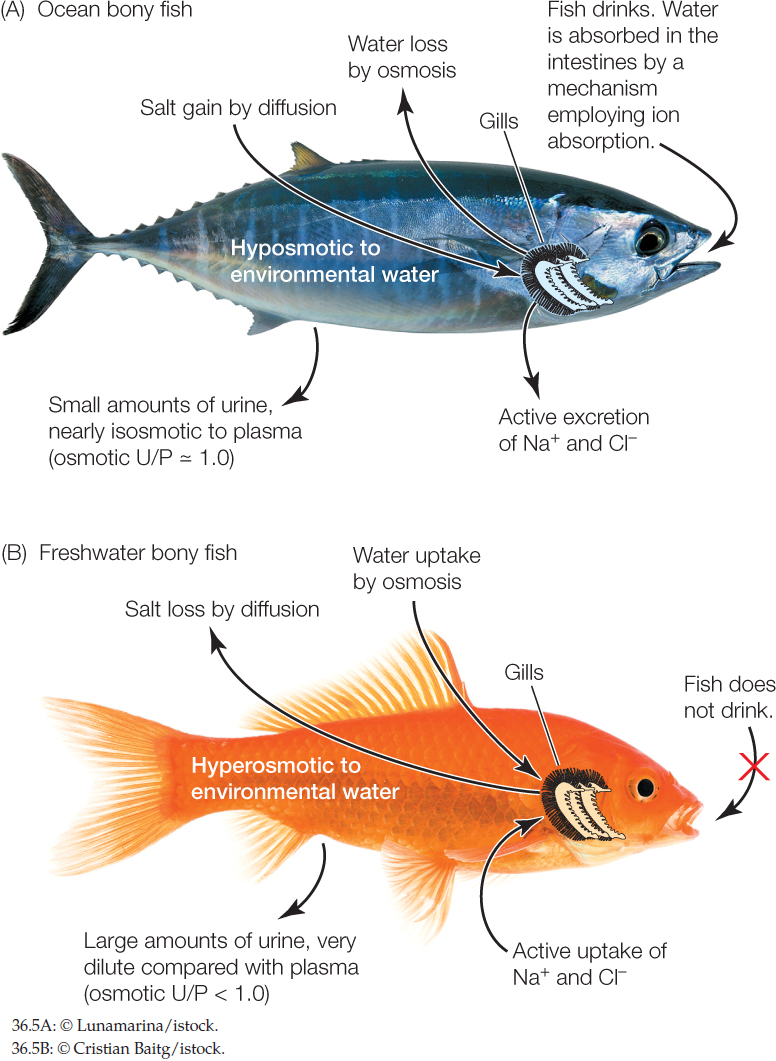
Ocean bony fish expend about 8–17 percent of their metabolic energy each day to fix these problems. To replace the water they are losing, they drink the seawater and expend energy in pumping ions across their intestinal wall so that water will enter their blood from their gut. They also use energy to excrete excess ions. The most concentrated urine their kidneys can produce (in terms of osmotic pressure) is U/P = 1.0. This is not sufficient to keep their body fluids more dilute than seawater. With inadequate kidneys, these fish have evolved vigorous extrarenal salt excretion. Specialized cells in their gills, known as mitochondria-rich cells or chloride cells, use ATP for active ion transport. These cells pump Na+ and Cl− out of the blood directly into the more concentrated seawater.
757
Why are the body fluids of these fish more dilute than seawater? Biologists do not know for certain. The preferred hypothesis today is that all the ocean bony fish evolved from freshwater ancestors, and that their dilute body fluids are a vestige of that earlier stage in their evolution.
All freshwater animals are hyperosmotic to fresh water
Animal life began in the oceans, as we’ve noted. During the long history of life, however, many phyla invaded rivers, streams, and lakes. Today’s freshwater invertebrates include crayfish, shrimp, clams, snails, and cnidarians such as hydra. In all these cases, the freshwater species are descended from ocean ancestors that had internal osmotic pressures near 1 Osm. The freshwater forms alive today, however, have internal osmotic pressures that are less than half that. Why?
An important general principle is that when the body fluids of an animal differ from the animal’s environment in some way, the cost of maintaining this difference tends to be greater when the difference is large than when it is small. (We are familiar with an analogy in our apartments and homes. The cost of keeping them warm in winter depends on how warm we keep them. Maintaining a big difference between the temperature inside and outside requires more energy than keeping the inside just slightly warmer than the outside.) When invertebrates first invaded fresh water from the ocean, they had a very large difference between their internal osmotic pressure, 1 Osm, and the external osmotic pressure, which is about 0 Osm in fresh water. Maintaining this difference required a high rate of energy use. By reducing their internal osmotic pressure over the course of evolutionary time, the animals lowered their rate of energy use. All are hyperosmotic to freshwater and are hyperosmotic regulators. Their cost of regulation is lower, however, than it would be if they had retained their ancestral internal concentrations.
Vertebrates have undergone similar changes. Biologists generally believe that the ancestors of today’s vertebrates were jawless fish, isosmotic with seawater, living in the ocean. They invaded fresh water, and over evolutionary time, their body fluids became more dilute. Probably they evolved an internal osmotic pressure of about 300–350 mOsm. At the same time, they evolved jaws. These early jawed fish with internal osmotic pressures near 300–350 mOsm diversified to give rise to today’s freshwater fish. They also invaded two other major habitats. Some reinvaded the oceans, giving rise to nearly all the ocean fish alive today, as noted in the last section. Others invaded the land.
LINK
For more on the evolutionary origins of terrestrial animals, see Concept 23.6
Because their body fluids are more concentrated than fresh water, all of today’s water-breathing freshwater animals—including fish, other water-breathing vertebrates, and invertebrates—face two relentless, never-ending problems that are the opposite of those faced by ocean fish (FIGURE 36.5B):
- They tend to gain water by osmosis from the dilute freshwater environment. Their steady gain of water tends to bloat them (recall that a goldfish gains water equal to about one-third of its body weight per day).
- They also tend to lose ions, such as Na+ and Cl−, by diffusion from their body fluids into their environment. Their steady gains of water and losses of ions tend to make their body fluids become too dilute.
Freshwater animals must invest energy to maintain their hyperosmotic state. To void the water they are gaining osmotically, their kidneys produce a large volume of urine per day. In most cases, the kidneys also produce urine that is far more dilute than the blood plasma (osmotic U/P < 1.0). Producing a large volume of dilute urine costs energy, but it means the kidneys help keep the blood at its correct osmotic pressure. Lost salts have to be replaced. Freshwater animals have specialized active-transport cells that use ATP to pump Na+ and Cl− directly from their freshwater environment into their blood. These cells are in the gills of fish, crayfish, and clams. They are in the skin of adult frogs. The pumps are so effective that they take up a lot of Na+ and Cl− per day even though these ions are exceedingly dilute in fresh water.
758
LINK
You can review active transport processes in Concept 5.3
Some aquatic animals face varying environmental salinities
Some fish—notably salmon, sturgeon, and many eels—have life cycles in which they migrate between rivers and the ocean. Here we’ll focus on salmon. They are conceived and undergo their early development in fresh water, complete much of their growth in the ocean, and finally return to fresh water to spawn. They switch between being typical hyperosmotic regulators when in fresh water to being typical hyposmotic regulators when in seawater (see Figure 36.5):
- A salmon in fresh water does not drink because it is already overloaded with water from osmosis. Upon entering the ocean, it starts to drink and activates intestinal ion transport mechanisms required to take water into its blood from the gut.
- When a salmon is in fresh water, its gills use ATP to pump ions into its blood from the surrounding water. In the ocean, its gills use ATP to pump ions out of its blood into the seawater.
- In fresh water, a salmon’s kidneys produce abundant urine that is very dilute compared with the blood plasma (osmotic U/P < 1.0). In seawater, the kidneys produce just a small volume of urine that has about the same osmotic pressure as the blood plasma (osmotic U/P = 1.0).
These changes are controlled by hormones such as prolactin.
Many nonmigratory aquatic animals face a range of environmental options. For example, consider ocean invertebrates. They might move into coastal waters where seawater mixes with fresh water and salinities are intermediate. They might even move into freshwater streams.
Most marine invertebrates are osmotic conformers (osmoconformers), but a minority are osmotic regulators (osmoregulators). In osmotic conformers, the osmotic pressure of the body fluids is always the same as that of the environmental water. Thus when osmotic conformers enter more dilute water, their body fluids become more dilute to the same extent. We know this is stressful because most of these animals do not survive in water that is much more dilute than seawater. Marine mussels are an exception. They prosper at a wide range of salinities even though they are osmotic conformers (FIGURE 36.6).
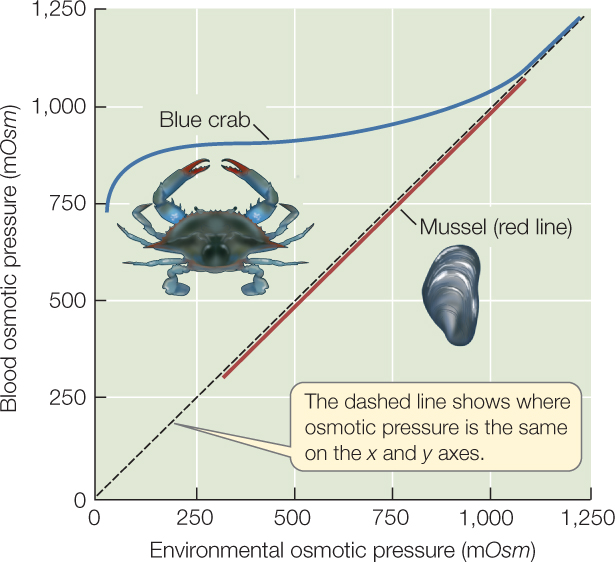
Most marine invertebrates that prosper in dilute waters, including the blue crabs (Callinectes sapidus) that are abundant in the coastal waters of our east and Gulf coasts, are osmotic regulators. When living in the ocean, they are nearly isosmotic with seawater, but they are hyperosmotic regulators when in dilute waters, keeping their blood osmotic pressure higher than the environmental osmotic pressure (see Figure 36.6). They thus invest energy in osmotic regulation. In return, they have a more stable internal osmotic pressure than conformers, and this stability allows them to be successful over a broad range of external salinities.
LINK
The general principles of regulation versus conformity are discussed in Concept 29.3, along with specific coverage of thermal relationships of animals with their environment
CHECKpointCONCEPT36.3
- Some marine mussels are of exceptional commercial importance, being considered gourmet foods. From the viewpoint of water and salt balance, in what way are mussels exceptional?
- Do bony fish living in the ocean steadily gain or lose water by osmosis from the seawater, or do they neither gain nor lose in a steady manner? Explain.
- In what way do the bony fish living in the ocean exhibit extrarenal salt excretion?
759
Now that we’ve seen how various animals living in aquatic environments deal with fresh water and with seawater, let’s turn to animals living on land.