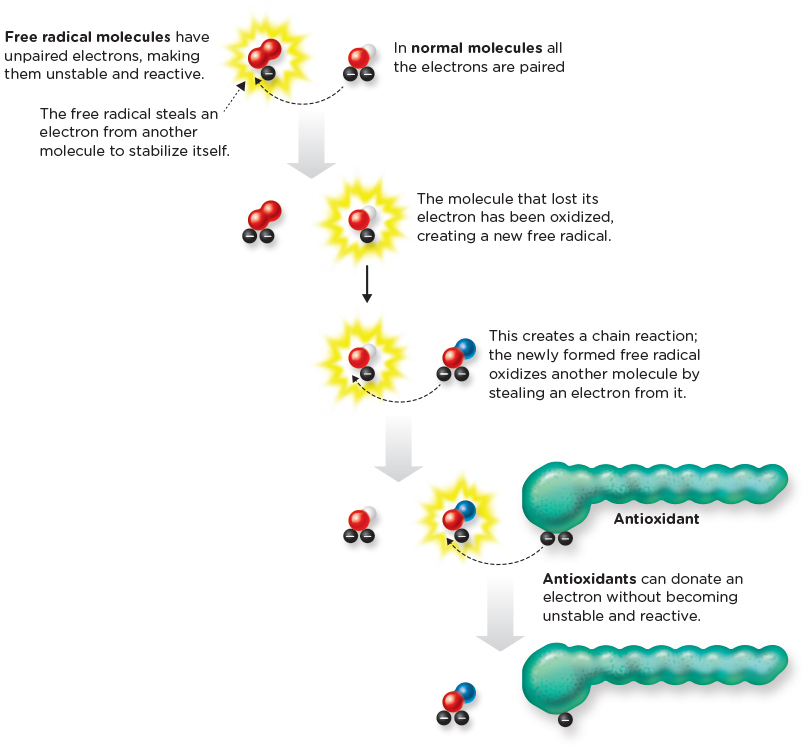
Figure INFOGRAPHIC 10.8 Antioxidants Defend Against Oxidative Damage Caused by Free Radicals Free radicals are molecules containing unpaired electrons. The highly reactive unpaired electron either causes oxidative damage to a molecule or is passed from molecule to molecule turning the recipient into a free radical and neutralizing the donor. Antioxidants are able to stop the chain reaction, by donating an electron.