IRON
As you saw in Infographic 14.1, iron (Fe) is the most abundant of the trace minerals in our body. Iron is a crucial component of hundreds of enzymes and other proteins in our body.
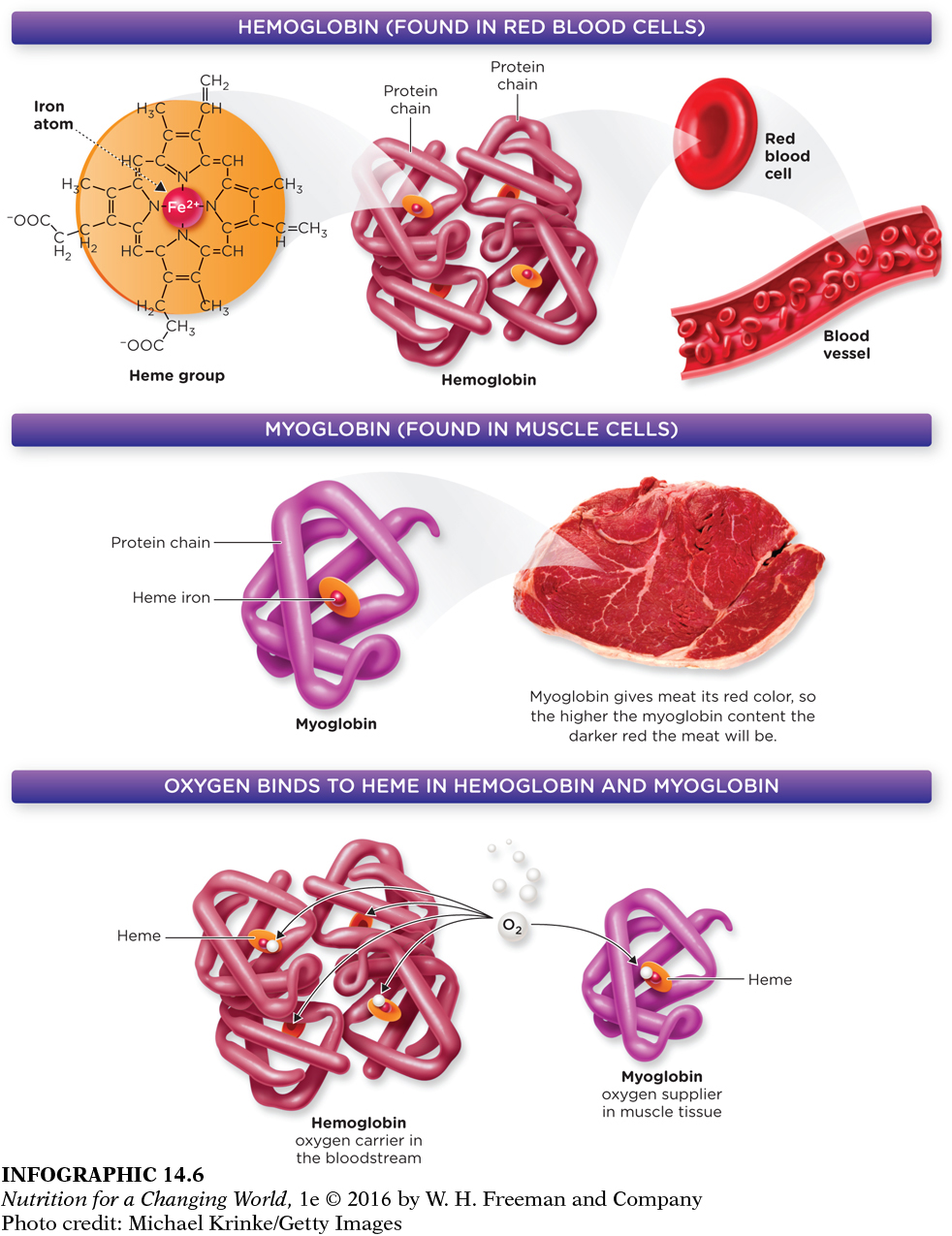
HEME IRON iron derived from hemoglobin; the most bioavailable form of dietary iron found in meat, fish and poultry
HEMOGLOBIN a protein in red blood cells that contains iron and carries oxygen to tissues
MYOGLOBIN a protein that functions to provide oxygen to muscles; contains less iron than hemoglobin
Most iron in our body occurs as heme iron. Heme iron is a critical part of the protein hemoglobin. In the body, red blood cells pick up oxygen from the lungs and release it into the tissues. Red blood cells are able to pick up and transport oxygen because they contain a protein within them called hemoglobin. Hemoglobin is made up of four units, each unit contains one heme group (an iron atom surrounded by a ring-
Question 14.2
 What type of chicken meat would likely have the most heme iron?
What type of chicken meat would likely have the most heme iron?
Dark chicken meat, such as the type found in a chicken leg, contains the most heme iron. The higher the myoglobin content, the darker the meat will be, and myoglobin contains heme iron.
In addition, iron is also an integral component of many enzymes required for a host of crucial processes in the body, including energy metabolism, protection against oxidative damage, the immune response, and DNA synthesis. Because of its role in DNA synthesis, iron is required for a wide variety of critically important functions, including reproduction, growth, and healing.
NON-
Although most iron is in heme form in the body, non-

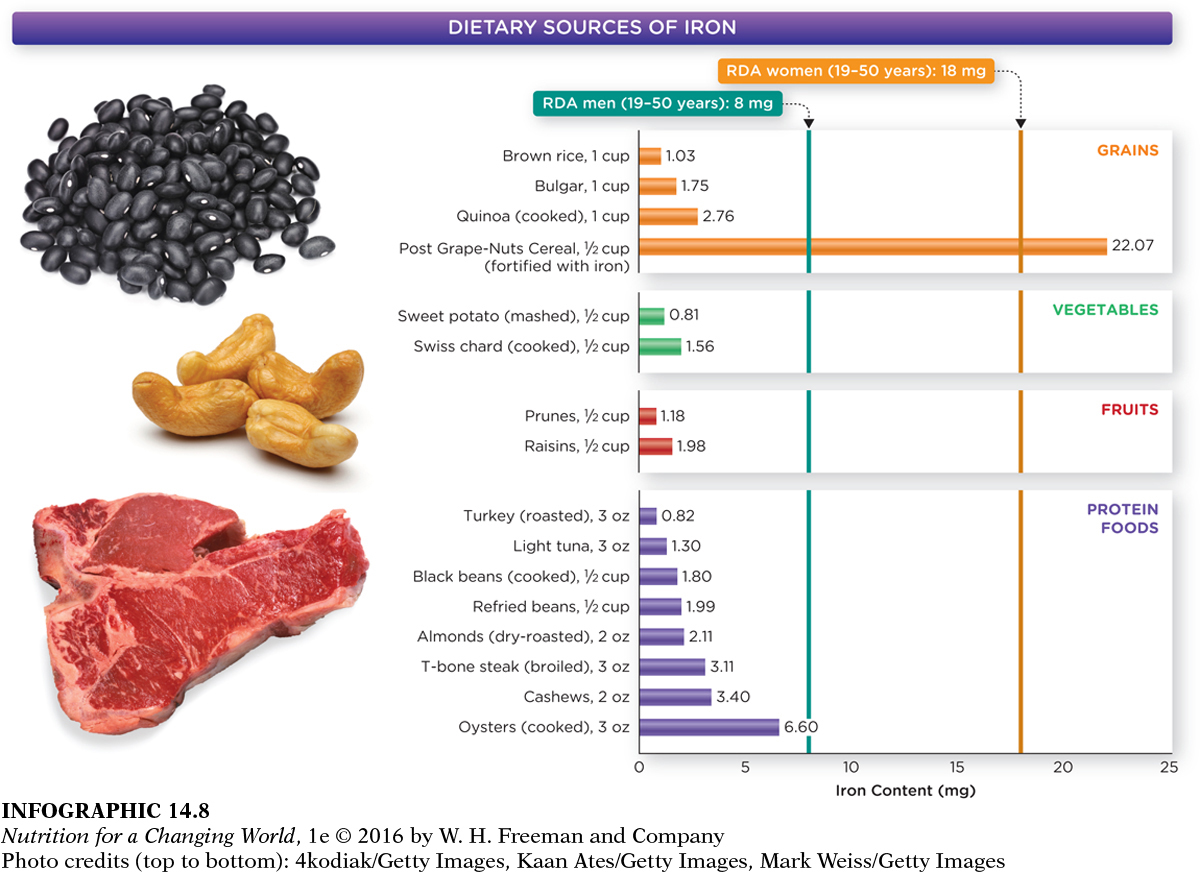
Food sources offer iron in both the heme and non-
Iron absorption
Non-

Iron stores in the body are regulated only by controlling its absorption in the small intestine, because, unlike other minerals, iron cannot be excreted in urine or bile. In healthy individuals about 10% to 15% of dietary iron is absorbed. However, when iron stores are low, iron is absorbed more efficiently, and when its stores are high, iron is absorbed less efficiently.
The body also carefully conserves iron so that daily losses are minimized. While we do lose iron through blood loss, the shedding of cells from the gastrointestinal tract and skin, and small losses in sweat, the body holds on to as much as possible. For example, when red blood cells die, iron is recycled and incorporated into new red blood cells. Iron balance in the body is achieved by carefully regulating iron absorption, storage, release, and transport. When imbalances develop, dangerous deficiencies and toxicities can occur.
Iron deficiency anemia
IRON-
As many as 30% of the world’s people may suffer from iron deficiency anemia (also known as microcytic hypochromic anemia), a serious condition that develops gradually when a person’s iron intake does not meet his or her daily needs. "Anemia" is a condition in which the oxygen carrying capacity of red blood cells is inadequate and "iron deficiency" is a cause of the type of anemia that results from a shortage of oxygen carrying hemoglobin. When iron intake is low over time, iron stores can become depleted; if iron stores become completely depleted, blood levels of iron fall, and hemoglobin synthesis is impaired. The decrease in hemoglobin synthesis results in less hemoglobin incorporation into red blood cells, and iron deficiency anemia develops.
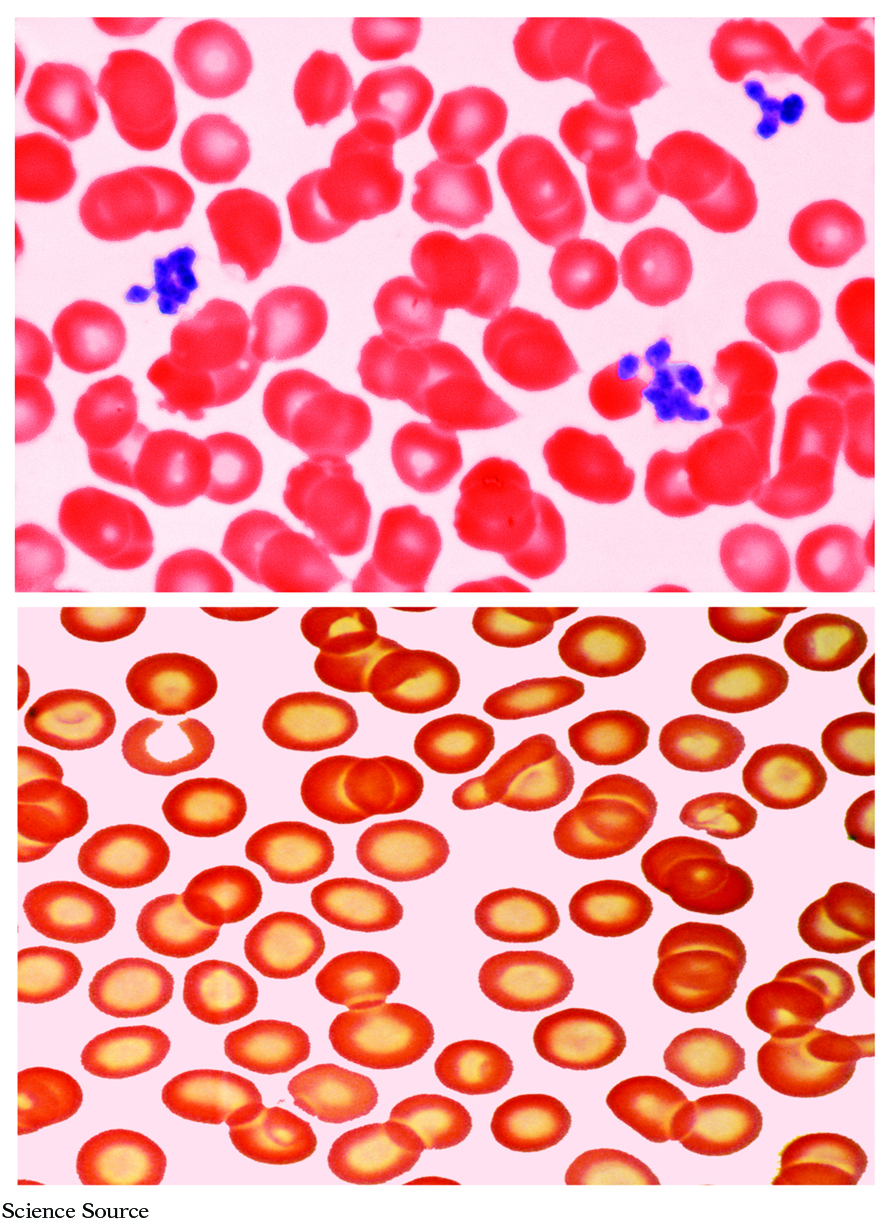
Iron deficiency anemia has many causes. The most common cause is blood loss. The combination of blood loss through menstruation and a limited or restricted diet that may minimize sources of heme iron can put premenopausal women at risk of iron-

People who suffer from iron deficiency anemia can have many symptoms. They can feel tired and out of breath, perform poorly at work or at school, and have slow cognitive and social development during childhood. They often have trouble maintaining their body temperature and are more susceptible to infections because their immune systems are not working properly. Iron deficiency anemia during pregnancy increases the risk of preterm birth and low birth weight, increasing the infants’ risk of health problems later. Individuals thought to be suffering from this form of anemia should be evaluated and managed by a health care provider, who will try to determine the cause of the condition and may prescribe iron supplements.
Iron intake recommendations
The RDA for iron for men 19 years and older is 8 mg; for women aged 19 to 50 years, it is 18 mg. However, these recommendations assume that 75% of iron consumption is heme iron, so for vegans and vegetarians, who primarily ingest non-
The UL for iron for men and women 19 years and older is 45 mg, and higher intakes of iron can cause gastrointestinal distress. Individuals who take high doses of iron to prevent or treat iron deficiency anemia also sometimes suffer from side effects, including constipation, nausea, vomiting, and diarrhea, especially when the supplements are taken on an empty stomach. Because very little iron is excreted from the body, iron toxicity can occur when intake is too high, causing symptoms such as apathy, fatigue, liver damage, and immune problems.
In children, iron poisoning is a major cause of unintentional poisoning death, causing symptoms such as nausea, vomiting, diarrhea, constipation, rapid heartbeat, dizziness, shock, and confusion (which explains why you will not find iron in “gummy” multivitamin/mineral supplements; children might mistake them for candy and consume too many with potentially fatal results). Men are at a higher risk for iron toxicity than are women because men don’t experience monthly blood loss. Individuals with hereditary hemachromatosis, sometimes called iron overload disease, are also at high risk of iron toxicity because of increased absorption and high iron stores.
■ ■ ■
In 1958, a young physician named Ananda Prasad evaluated a patient in Iran who suffered from severe iron deficiency and its associated symptoms. In addition, the severely stunted patient appeared to be about eight years old and had not gone through puberty, although his chronological and bone age indicated he was closer to 21 years old. This was not an isolated case; the same condition was so prevalent in Iran that it was considered an epidemic.
Dr. Prasad studied the curious problem, administering iron to the patients with iron deficiency. However, Prasad found it difficult to assign all the health problems to iron deficiency, since growth retardation and the lack of secondary sex characteristics are not typically linked to iron deficiency.
Seeking clues to effectively treat the patients, Prasad went to Egypt to study rural farmers with similar signs of illness, taking careful inventory of their diets. Soon, Prasad began to target the mineral zinc, which, at that time, was not thought to be of importance to human health. He knew that in the developed world, zinc is found in a variety of food sources, such fish, red meat, and dairy products. However, the diets of the individuals he studied from the developing world relied heavily on breads and grains, which contain phytates, which are substances that bind zinc and iron and prevent both minerals from being properly absorbed, accounting for both the iron deficiency and the zinc deficiency.
In 1961, Dr. Prasad published an article in the American Journal of Medicine, suggesting for the first time that zinc deficiency could account for human growth retardation. By 1963, Dr. Prasad started administering zinc through clinical trials, and his participants began growing taller and developing secondary sex characteristics.