Chapter 1. Impact 15.2
Impact …ON TECHNOLOGY: I15.2 Fuel cells
A fuel cell operates like a conventional galvanic cell with the exception that the reactants are supplied from outside rather than forming an integral part of its construction. A fundamental and important example of a fuel cell is the hydrogen/oxygen cell, such as the ones used in space missions (Fig. 1). One of the electrolytes used is concentrated aqueous potassium hydroxide maintained at 200 °C and 20–40 atm; the electrodes may be porous nickel in the form of sheets of compressed powder. The cathode reaction is the reduction
O2(g) + 2 H2O(l) + 4 e− → 4 OH−(aq) E⊖ = +0.40 V
and the anode reaction is the oxidation
H2(g) + 2 OH−(aq) → 2 H2O(l) + 2 e−
For the corresponding reduction, E⊖ = −0.83 V. Because the overall reaction
2 H2(g) + O2(g) → 2 H2O(l) E⊖cell = +1.23 V
is exothermic as well as spontaneous, it is less favourable thermodynamically at 200 °C than at 25 °C, so the cell potential is lower at the higher temperature. However, the increased pressure compensates for the increased temperature, and E ≈ +1.2 V at 200 °C and 40 atm.
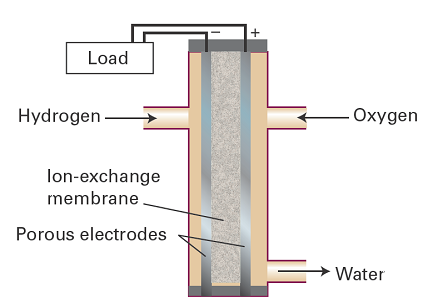
Fig. 1. A single cell of a hydrogen/oxygen fuel cell. In practice, a stack of many cells is used.
One advantage of the hydrogen/oxygen system is the large exchange current density of the hydrogen reaction. Unfortunately, the oxygen reaction has an exchange current density of only about 0.1 nA cm−2, which limits the current available from the cell. One way round the difficulty is to use a catalytic surface (to increase j0) with a large surface area. One type of highly developed fuel cell has phosphoric acid as the electrolyte and operates with hydrogen and air at about 200 °C; the hydrogen is obtained from a reforming reaction on natural gas:
Anode: 2 H2(g) → 4 H+(aq) + 4 e−
Cathode: O2(g) + 4 H+(aq) + 4 e− → 2 H2O(l)
This fuel cell has shown promise for combined heat and power systems (CHP systems). In such systems, the waste heat is used to heat buildings or to do work. Efficiency in a CHP plant can reach 80 per cent. The power output of batteries of such cells has reached the order of 10 MW. Although hydrogen gas is an attractive fuel, it has disadvantages for mobile applications: it is difficult to store and dangerous to handle. One possibility for portable fuel cells is to store the hydrogen in carbon nanotubes. It has been shown that carbon nanofibres in herringbone patterns can store huge amounts of hydrogen and result in an energy density (the magnitude of the released energy divided by the volume of the material) twice that of gasoline.
Cells with molten carbonate electrolytes at about 600 °C can make use of natural gas directly. Solid-state electrolytes are also used. They include one version in which the electrolyte is a solid polymeric ionic conductor at about 100 °C, but in current versions it requires very pure hydrogen to operate successfully. Solid ionic conducting oxide cells operate at about 1000 °C and can use hydrocarbons directly as fuel. Until these materials have been developed, one attractive fuel is methanol, which is easy to handle and is rich in hydrogen atoms:
Anode: CH3OH(l) + 6 OH−(aq) → 5 H2O(l) + CO2(g) + 6 e−
Cathode: O2(g) + 2 H2O(l) + 4 e− → 4 OH−(aq)
One disadvantage of methanol, however, is the phenomenon of ‘electro-osmotic drag’ in which protons moving through the polymer electrolyte membrane separating the anode and cathode carry water and methanol with them into the cathode compartment where the potential is sufficient to oxidize CH3OH to CO2, so reducing the efficiency of the cell.