Chapter 1. Impact 19.1
Impact …ON BIOCHEMISTRY: I19.1 Harvesting of light during plant photosynthesis
A large proportion of solar radiation with wavelengths below 400 nm and above 1000 nm is absorbed by atmospheric gases such as ozone and O2, which absorb ultraviolet radiation, and CO2 and H2O, which absorb infrared radiation (Impact I9.2). As a result, plants, algae, and some species of bacteria evolved photosynthetic apparatus that capture visible and near-infrared radiation. Plants use radiation in the wavelength range of 400–700 nm to drive the endergonic reduction of CO2 to glucose, with concomitant oxidation of water to O2 (ΔrG⊕ = +2880 kJ mol-1, the superscript ⊕ denotes a biological standard state) in essence the reverse of glycolysis and the citric acid cycle (Impact I15.1):
6 CO2(g) + 6 H2O(l) ⇄ C6H12O6(aq) + 6 O2(g)
Electrons flow from reductant to oxidant in a series of electrochemical reactions that are coupled to the synthesis of ATP. The process takes place in the chloroplast, a special organelle of the plant cell, where chlorophylls a and b (1) and carotenoids (of which β-carotene, 2, is an example) bind to integral proteins called light harvesting complexes, which absorb solar energy and transfer it to protein complexes known as reaction centres, where light-induced electron transfer reactions occur. Working together, the light harvesting complexes and reaction centres of plants drive the reduction of NADP+ (3) by water:
2 H2O + 2 NADP+ \underrightarrow {\mathrm {Light}} O2 + 2 NADPH + 2 H+
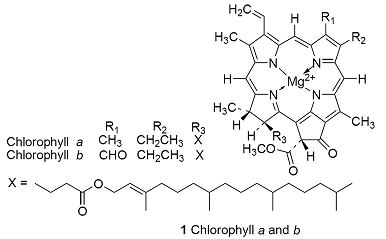
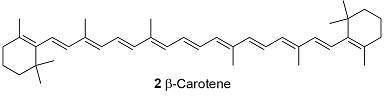
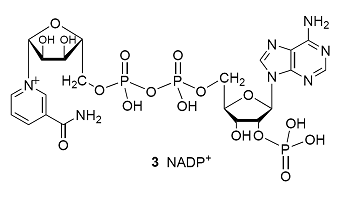
It is clear that energy from light is required to drive this reaction because, in the dark, E\oplus= –1.135 V and \DeltarG\oplus = +438.0 kJ mol–1.
Light harvesting complexes bind large numbers of pigments in order to provide a sufficiently large area for capture of radiation. Absorption of a photon raises a chlorophyll or carotenoid molecule to an excited singlet state and within 0.1–5 ps the energy hops to a nearby pigment by the Förster mechanism. About 100–200 ps later, which corresponds to thousands of hops within the light harvesting complex, more than 90 per cent of the absorbed energy reaches the reaction centre. There, a chlorophyll a dimer becomes electronically excited and initiates an ultrafast electron transfer reaction that occur within a few picoseconds. Once the excited state of the chlorophyll dimer has been quenched efficiently by this first reaction, subsequent steps that lead to the oxidation of water and reduction of NADP+ occur more slowly, with reaction times varying from 200 ps to 1 ms. We see that the initial energy and electron transfer events of photosynthesis are under tight kinetic control. Photosynthesis captures solar energy efficiently because the excited singlet state of chlorophyll is quenched rapidly by processes that occur with relaxation times that are much shorter than the fluorescence lifetime, which is typically about 1 ns in organic solvents at room temperature.
In summary, plant photosynthesis uses solar energy to transfer electrons from a poor reductant (water) to carbon dioxide. In the process, high energy molecules (carbohydrates, such as glucose) are synthesized in the cell. Animals feed on the carbohydrates derived from photosynthesis. During aerobic metabolism, the O2 released by photosynthesis as a waste product is used to oxidize carbohydrates to CO2, driving biological processes, such as biosynthesis, muscle contraction, cell division, and nerve conduction. Hence, the sustenance of life on Earth depends on a tightly regulated carbon–oxygen cycle that is driven by solar energy.