Chapter 1. Impact 9.3
Impact …ON BIOCHEMISTRY: I9.3 Vision
The eye is an exquisite photochemical organ that acts as a transducer, converting radiant energy into electrical signals that travel along neurons. Here we concentrate on the events taking place in the human eye, but similar processes occur in all animals. Indeed, a single type of protein, rhodopsin, is the primary receptor for light throughout the animal kingdom, which indicates that vision emerged very early in evolutionary history, no doubt because of its enormous value for survival.
Photons enter the eye through the cornea, pass through the ocular fluid that fills the eye, and fall on the retina. The ocular fluid is principally water, and passage of light through this medium is largely responsible for the chromatic aberration of the eye, the blurring of the image as a result of different frequencies being brought to slightly different focuses. The chromatic aberration is reduced to some extent by the tinted region called the macular pigment that covers part of the retina. The pigments in this region are the carotene-like xanthophylls (1), which absorb some of the blue light and hence help to sharpen the image. They also protect the photoreceptor molecules from too great a flux of potentially dangerous high energy photons. The xanthophylls have delocalized electrons that spread along the chain of conjugated double bonds, and the π*←π transition lies in the visible.

About 57 per cent of the photons that enter the eye reach the retina; the rest are scattered or absorbed by the ocular fluid. Here the primary act of vision takes place, in which the chromophore of a rhodopsin molecule absorbs a photon in another π *←π transition. A rhodopsin molecule consists of an opsin protein molecule to which is attached a 11-cis-retinal molecule (2). The latter resembles half a carotene molecule, showing Nature’s economy in its use of available materials. The attachment is by the formation of a protonated Schiff’s base, utilizing the –CHO group of the chromophore and the terminal NH2 group of the sidechain, a lysine residue from opsin. The free 11-cis-retinal molecule absorbs in the ultraviolet, but attachment to the opsin protein molecule shifts the absorption into the visible region. The rhodopsin molecules are situated in the membranes of special cells (the ‘rods’ and the ‘cones’) that cover the retina. The opsin molecule is anchored into the cell membrane by two hydrophobic groups and largely surrounds the chromophore.
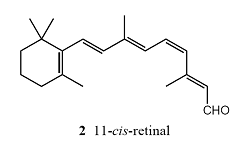
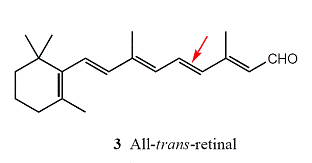
Immediately after the absorption of a photon, the 11-cis-retinal molecule undergoes photoisomerization into all-trans-retinal (3). Photoisomerization takes about 200 fs and about 67 pigment molecules isomerize for every 100 photons that are absorbed. The process occurs because the π *←π excitation of an electron loosens one of the π-bonds (the one indicated by the arrow in 3), its torsional rigidity is lost, and one part of the molecule swings round into its new position. At that point, the molecule returns to its ground state, but is now trapped in its new conformation. The straightened tail of all-trans-retinal results in the molecule taking up more space than 11-cis-retinal did, so the molecule presses against the coils of the opsin molecule that surrounds it. In about 0.25–0.50 ms from the initial absorption event, the rhodopsin molecule is activated both by the isomerization of retinal and deprotonation of its Schiff’s base tether to opsin, forming an intermediate known as metarhodopsin II.
In a sequence of biochemical events known as the biochemical cascade, metarhodopsin II activates the protein transducin, which in turn activates a phosphodiesterase enzyme that hydrolyses cyclic guanine monophosphate (cGMP) to GMP. The reduction in the concentration of cGMP causes ion channels, proteins that mediate the movement of ions across biological membranes, to close and the result is a sizable change in the transmembrane potential (see Impact I16.2 for a discussion of transmembrane potentials). The pulse of electric potential travels through the optical nerve and into the optical cortex, where it is interpreted as a signal and incorporated into the web of events we call ‘vision’.
The resting state of the rhodopsin molecule is restored by a series of nonradiative chemical events powered by ATP. The process involves the escape of all-trans-retinal as all-trans-retinol (in which –CHO has been reduced to –CH2OH) from the opsin molecule by a process catalysed by the enzyme rhodopsin kinase and the attachment of another protein molecule, arrestin. The free all-trans-retinol molecule now undergoes enzyme-catalysed isomerization into 11-cis-retinol followed by dehydrogenation to form 11-cis-retinal, which is then delivered back into an opsin molecule. At this point, the cycle of excitation, photoisomerization, and regeneration is ready to begin again.