Congratulations on completing this Case Study!
A final note and plea from the author
 Overall Progress: 6%
Overall Progress: 6%

Toxic Alcohols
By Justin Hines, Lafayette College

“Help!” The call was muffled; Emergency Room doors burst open.
“Help! My daughter—she drank antifreeze!!” A frantic man carrying a frightened child ran to the reception desk.
The triage nurse knew immediately what this meant and paged the attending physician.
“I need to know exactly what she swallowed and how much! We have to act fast,” she explained to the father. “Sweetie . . . , ” turning to the child and forcing a smile. “It’s very important that you tell me exactly what you drank and how long ago.”
The child, a girl of eight, was clearly scared. “It was green. I thought it was juice. I’m sorry!” she squealed, turning her head back into her father’s chest before sobbing.
“It’s my fault—” the man interrupted, visibly shaking. “I had it in a water bottle in the backseat of my car for emergencies. So stupid! I caught her drinking it and we came straight here, so it’s been about 20 minutes.” He pulled a nearly full water bottle of green liquid out of his jacket and handed it to the arriving doctor.
“Sir,” the doctor spoke quietly and away from the little girl, “this is very serious. Your daughter may have kidney failure in a matter of hours if we don’t treat her immediately. We need to pump her stomach and give her an ADH inhibitor. We may even need to consider dialysis. You may have saved her life by bringing her here so quickly but to treat her properly I need to know what, exactly, she drank. Can someone bring in the actual bottle?”
“Yes . . . yes! My brother lives up the street and has a key. He’s home. I’ll call him right now.”
The doctor nodded and turned to the girl, “Sweetie, it’s going to be OK. We’re going to give you some medicine and make you all better.”
The ER staff administered a dose of an alcohol dehydrogenase (ADH) inhibitor called Fomepizole. Just as the girl was about to undergo gastric lavage (“stomach pumping”), her uncle arrived with the original antifreeze container. The ER doctor closely examined the bottle and then turned to the girl’s father: “You’re sure that this is what she drank? How much again?”
“About a quarter of a cup I think, just what’s missing from the water bottle.”
A smile came across the doctor’s face as he turned toward the little girl, “Young Miss, you’re going to be just fine.” To the nurse and father: “We’ll cancel the lavage and dialysis and with the ADH inhibitor she’s already on, let’s just keep her drinking water to flush the remainder out of her system. Let’s monitor her for another hour here just to be certain, but then you can both go home.”
The father, simultaneously confused and relieved, interjected: “What? But— well that’s great! But Doc, how is it that this was so serious a minute ago and now we can just leave?”
The doctor pointed to the label on the bottle, “You see right here: it says ‘pet-safe antifreeze.’ The dose makes the poison, and this stuff isn’t that bad in small doses.”
Befuddled but profoundly relieved, the father exclaimed: “But it’s still antifreeze! What’s the difference??!!”
Many different toxic alcohols…
In this case, we explore the toxicity of several alcohols commonly encountered in daily life. In particular, we focus on the enzyme-catalyzed metabolic reactions in humans that largely determine the vast differences in alcohol toxicities. We have already been introduced to antifreeze and so-called “pet-safe” antifreeze. The main component of each is an alcohol, but the alcohol of regular antifreeze and the alcohol of “pet-safe” antifreeze have vastly different toxicities. Note that the old adage “the dose makes the poison” is a reference to the biochemical fact that nothing is toxic at low enough concentrations, and everything is toxic at high enough concentrations, even water! What distinguishes these compounds are the relative amounts of each that the human body can tolerate.
To see what we mean, let’s first take a look at five common alcohols encountered in daily life:
Ethanol
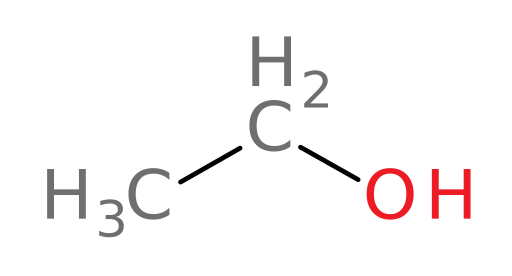
Isopropanol
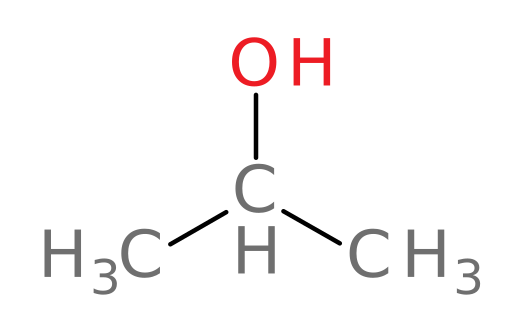
Methanol
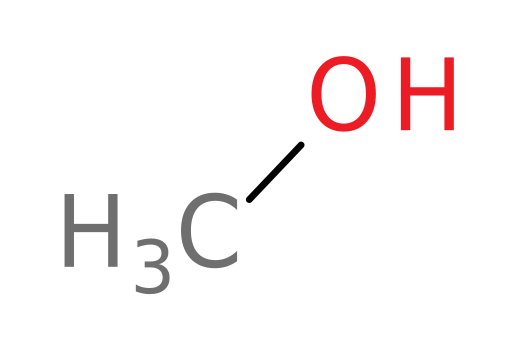
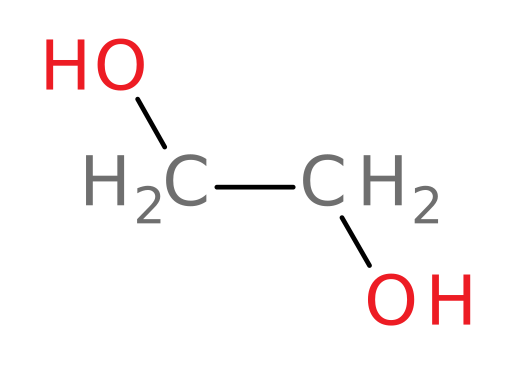
Ethylene glycol
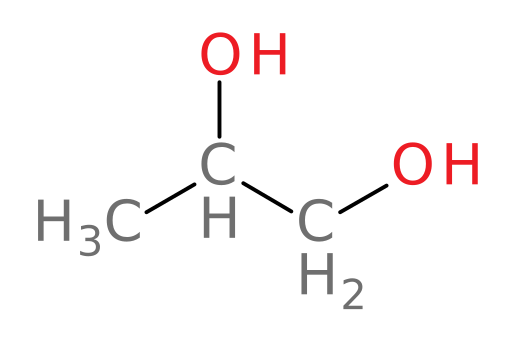
Propylene glycol

Rating relative alcohol toxicity
How much do you know about these compounds? Can you rank them in descending order of acute toxicity to humans? Try ranking them in the question below. (Note: This question is not scored, but is just a starting point to test your knowledge.)
On a separate piece of paper rank the following compounds in order of decreasing acute human toxicity, from most toxic to least toxic:
ethanol
isopropanol
methanol
ethylene glycol
propylene glycol

Here is the actual order:
methanol > ethylene glycol > isopropanol > ethanol > propylene glycol
How well did you do? Here is where these compounds are commonly found:
ethanol (grain alcohol): found in “alcoholic beverages” and often used in gasoline
isopropanol (rubbing alcohol): used as an antiseptic agent and a common organic solvent
methanol (wood alcohol): an undesirable byproduct of some fermentation processes and a common organic solvent
ethylene glycol: the primary component of traditional antifreeze
propylene glycol: the primary component of “pet-safe” antifreeze and a food and cosmetic additive

LD50 values for commonly encountered alcohols
One way to express the relative toxicity of a compound is to determine an LD50 (Lethal Dose 50%) value. The LD50 is the concentration that is expected to kill 50% of the sample population. LD50 values can only be determined experimentally using living organisms. As you might expect, LD50 values are hard to know for human beings and are almost always estimated from data obtained using model organisms (often bacteria, yeast, fruit flies, plants, and laboratory animals). The following LD50 values are estimates based on available animal data. LD50 values are usually given in milligrams per kilogram of body weight. To make these values easier to understand, here they are given in milliliters of pure liquid and for the “average” American who is 175 pounds. Values scale to body mass, so they are higher for more massive individuals and lower for less massive individuals:
| Compound | Estimated LD50 value |
| 1. methanol | ~60 mL |
| 2. ethylene glycol | ~120 mL |
| 3. isopropanol | ~250 mL |
| 4. ethanol | ~600 mL |
| 5. propylene glycol | ~1500 mL |
| 6. water | ~4000 mL |
Note: Again, these LD50 values are estimates made primarily from available animal data and for an average American who weighs 175 pounds. It would be profoundly unethical to actually conduct an experiment to determine an LD50 value for a human so no one actually knows the real values! These are not exact figures and in no way should they be used to infer a safe level of consumption for these compounds. That said, we can compare relative toxicities using these values. Note that methanol and ethylene glycol are the most toxic and propylene glycol is the least toxic. Why is water included? It’s included to reinforce the point that everything is toxic at a high enough concentration (the dose makes the poison). Drinking too much water too quickly dilutes ion concentrations in bodily fluids, disrupting oxygen transport and nerve impulses, and can result in death from “water intoxication”. Knowing this, how do you go about defining a “toxin”?

What distinguishes the toxicity of different alcohols?
The answer has less to do with the alcohol itself and more to do with how the human body metabolizes each alcohol. That is, the five alcohols we listed are similarly toxic on their own, but some are quickly converted to less toxic compounds, while others are converted to more toxic compounds. Let’s learn more about how the human liver detoxifies potentially dangerous molecules that we ingest in our diet.
First, let’s remember that the entire digestive tract is a hollow passageway that passes through the human body, but that the interior of the tract itself—the esophagus, stomach, intestines, etc.—is actually outside the human body. When you ingest something, molecules are absorbed into the body from the various parts of the digestive tract, but particularly throughout the small intestine. These molecules then enter the bloodstream. Where does this blood go first? The venous blood from the intestinal tract flows directly into the liver, allowing the liver to serve as a first-pass filter that protects the rest of the body from the impact of many dangerous substances that may have been ingested. As such, the liver has an active role in detoxifying many compounds that human beings commonly ingest. These detoxification reactions are all catalyzed by various proteins, called enzymes, made in the liver. Before progressing any further, please pause to read and/or review Sections 8.1-8.3 on pages 216-225 of Stryer Biochemistry, 8th ed. to familiarize yourself with the fundamentals of enzyme function. Once you’ve done so, please answer the following questions:
Which of the following statements is true?
| A. |
| B. |
| C. |
| D. |
| E. |
| F. |
Which of the following is NOT a true statement about the role of enzymes in catalysis?
| A. |
| B. |
| C. |
| D. |
| E. |
An enzyme’s active site will ultimately form a shape that is most complimentary to which of the following structures?
| A. |
| B. |
| C. |

Why oxidize?
Liver enzymes oxidize many compounds as the primary method of detoxification within the body. General Chemistry Reminder: Remember that oxidation is the loss of electrons, not necessarily a reaction with oxygen. Oxidation has two purposes in this context: (1) it usually increases the water solubility of the compound, aiding the elimination of the compound from the body in the urine and (2) it often results in the production of a less toxic compound. As we’ll see in this case study, this second purpose of oxidation backfires in some cases because the product molecule, called a metabolite, is sometimes more toxic than the original molecule!
Chemistry Tutorial: The oxidation of alcohols
The oxidation of alcohols can be monitored by determining the oxidation state of the carbon atom attached to the oxygen atom within molecules, both before and after reactions.
General Chemistry Reminder: The oxidation state (Ox) of an atom is the hypothetical difference between the number of valence electrons (Eval) that the neutral atom would have and the number of electrons that “belong” to that atom (Eown) in the molecular structure using the following guidelines: electrons engaged in a bond between atoms “belong” to the more electronegative atom or are split evenly if the two atoms are the same. Therefore, Ox = Eval - Eown.
See page 432, Figure 15.9 of Stryer Biochemistry, 8th ed., for additional examples of carbon compounds in various stages of oxidation (depicted left to right from least oxidized to most oxidized) and see the examples below.
For example, the following compound (1-propanol) can be metabolized by the liver enzyme alcohol dehydrogenase (ADH):

The carbon attached to the hydroxyl group is the atom that will be oxidized in this reaction, as we will see shortly. Let’s draw this part of the molecule as a Lewis dot structure and attribute the electrons in the covalent bonds to the most electronegative atom (approximate electronegativities are as follows: H = 2.1, C = 2.5, and O = 3.5):
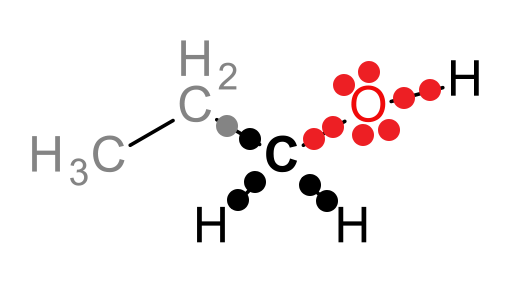
Look at the black carbon. This is the atom that will be oxidized and the one for which we need to determine the oxidation state. In the structure above, 5 electrons belong to the carbon atom and 8 electrons belong to the oxygen atom. A neutral carbon atom should have 4 valence electrons, and thus, the oxidation state (Eval - Eown) of the carbon of interest is: 4 – 5 or -1.
If the compound is being oxidized, we expect the oxidation state of the carbon to increase as electrons are removed. Let’s look at the oxidation state of the carbonyl carbon in the product and compare it to that of the reactant. Using the same method, we get the following structure of the product:
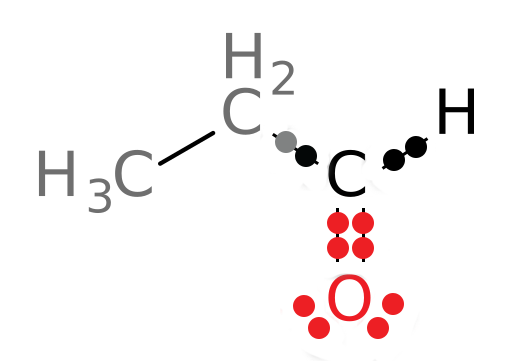
In this structure, 3 electrons now belong to the carbonyl carbon atom. The oxidation state of this carbon is: 4-3 or +1. We see that the oxidation state of the carbon atom of interest has increased as expected: The carbon has been oxidized since it now has 3 electrons attributed to it in the structure whereas it used to have 5. Its oxidation state has increased from -1 to +1. Organic Chemistry Reminder: Be sure to note that, as you learned in organic chemistry, primary alcohols like the reactant in the above reaction can be oxidized to aldehydes, like the product of the reaction. This is one role of ADH: It oxidizes primary alcohols to aldehydes.
The product of the above reaction is called propyl-aldehyde (or propionaldehyde) and it can be further metabolized by a second liver enzyme aldehyde dehydrogenase (ADLH).

Again, if the compound is being oxidized, we expect the oxidation state of the carbon atom of interest to increase. Using the same method, we get the following structure of the product:
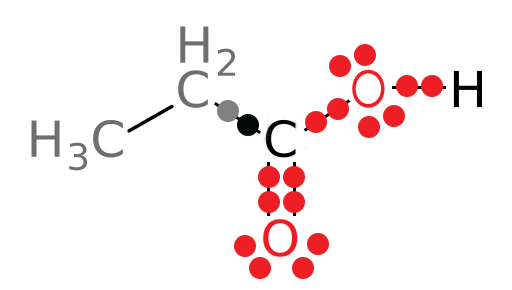
What type of organic molecule is the product of the above reaction? (Hint: If you do not immediately recall this type of organic molecule, you may want to use an organic chemistry textbook, or a website, to briefly review organic functional groups before you continue. You may use web resources as a reference as necessary as you continue with the case. One fairly large listing of organic functional groups can be found here: https://en.wikipedia.org/wiki/Functional_group)
| A. |
| B. |
| C. |
| D. |
| E. |
What is the oxidation state of the carboxyl carbon in the above product structure?
| A. |
| B. |
| C. |
| D. |
| E. |

Promiscuous enzymes metabolize many alcohols in the human liver
The example molecule used in the Chemistry Tutorial (1-propanol) and its metabolite are not the only molecules that can be oxidized by ADH and ALDH, respectively. In fact, ADH and ALDH are promiscuous enzymes, meaning that they are not specific in catalyzing the oxidation of just one specific alcohol or aldehyde. On the other hand, ADH will not catalyze the oxidation of every alcohol either.
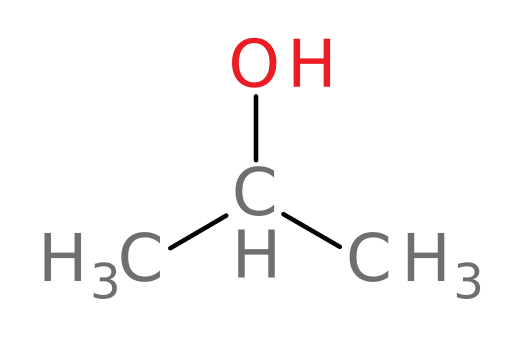
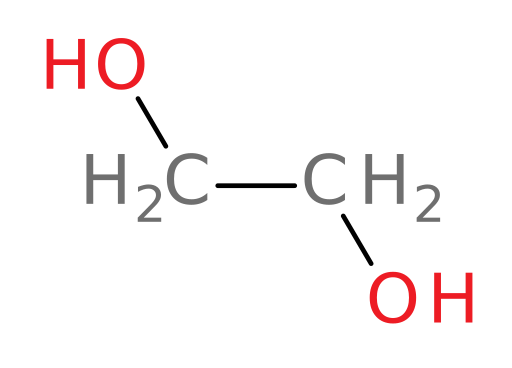
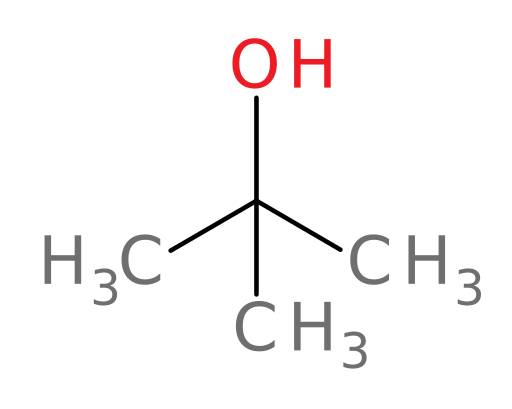
For example, ADH can oxidize all of the following compounds except tert-butanol.
methanol

isopropanol
ethylene glycol
tert-butanol
What quality of the substrate must the active site of ADH recognize?
| A. |
| B. |
| C. |
| D. |
As the name implies, the active site of aldehyde dehydrogenase (ALDH) recognizes aldehydes as opposed to alcohols. Just like ADH, ALDH’s active site also recognizes a hydrogen atom attached to the carbon to be oxidized, geminal to the carbonyl group. Note that a hydrogen geminal to a carbonyl is the definition of an aldehyde! Therefore, ALDH can potentially oxidize any aldehyde to a carboxylic acid, as we will see in just a moment.
Why the need for hydrogen? A hydrogen atom in the correct position is needed because, like many biochemical oxidation-reduction reactions, these reactions are catalyzed by “dehydrogenases.” Dehydrogenases typically remove a pair of electrons and a proton (H+); together, the proton and electrons are known as a hydride ion (H-). The hydride ion is typically transferred to an electron carrier molecule, usually NAD+, to form NADH. Thus, for most dehydrogenases, NAD+ is a cosubstrate and NADH is a product. For example, ADH uses NAD+ to oxidize ethanol, producing acetylaldehyde and NADH. Note that because electrons are transferred from ethanol to NAD+, ethanol is oxidized and NAD+ is reduced. To keep things simple and focused, however, we have omitted showing these and any other cosubstrates and products in our reactions.
As discussed above, ADH metabolizes alcohols, as long as a hydrogen geminal to the hydroxyl group is present; this hydrogen, in the form of a hydride ion, will be removed. Thus, ADH metabolizes alcohols in the following manner:
Primary alcohols are oxidized to form aldehydes:

Secondary alcohols are oxidized to form ketones:

And tertiary alcohols are not metabolized due to the lack of a hydrogen in the correct position:
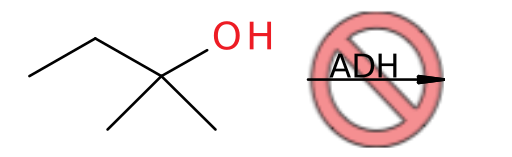
As previously mentioned, ALDH only metabolizes aldehydes, but it can potentially oxidize any aldehyde to a carboxylic acid in the following manner:

Similar to the inability of ADH to act upon tertiary alcohols, ALDH will not metabolize ketones because there is no hydrogen geminal to the oxygen to remove:

Because secondary alcohols will be oxidized to ketones by ADH, they cannot be subsequently metabolized by ALDH. Consequently, only primary alcohols can be metabolized by both ADH and ALDH in succession.

Metabolism of isopropanol
With the information you’ve learned so far, let’s see if you’ve got the hang of how ADH and ALDH act to metabolize alcohols. We will begin by examining isopropanol (rubbing alcohol). Feel free to refer back to the tutorial as you progress.
ADH metabolizes isopropanol (structure shown below) into which of the following compounds?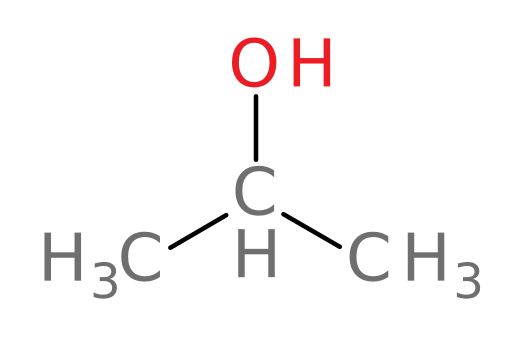
| A. |
| B. |
| C. |
| D. |
| E. |
Will ALDH further metabolize the product of the previous reaction?
| A. |
| B. |
| C. |

Metabolism of ethanol
ADH metabolizes ethanol (structure shown below) into which of the following compounds?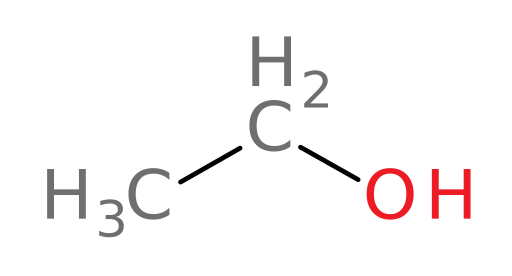
| A. |
| B. |
| C. |
| D. |
| E. |
The final product of the previous question is acetaldehyde (see previous question for structure). Acetaldehyde is then further metabolized by aldehyde dehydrogenase (ALDH) into which product?
| A. |
| B. |
| C. |
| D. |
| E. |
Can we obtain nutritional Calories from ethanol? The acetic acid from ethanol metabolism is converted into acetyl-CoA, a central metabolite, which is further metabolized for energy. Thus, alcohol is a food substance, providing humans with substantial caloric energy. In fact, it provides 7 Cal/g, more than protein and even more than carbohydrates (both 4 Cal/g). General Chemistry Reminder: Do you recall the difference between a calorie (cal) and a Calorie (Cal)? The latter is also called a ”nutritional calorie” and is equivalent to a kilocalorie or 1000 calories. Thus, enough energy is released from the metabolism of just one gram of ethanol to heat 7 liters of water (nearly two gallons) by one degree Celsius. Just 3 grams of ethanol has enough chemical energy to bring a cup of water from room temperature to boiling! Ethanol is actually most similar in calories to fat, which provides 9 Cal/g. From a caloric intake perspective, drinking ethanol is similar to drinking pure olive oil!
Why do we have these enzymes for metabolizing alcohol in the liver? In the time scale of human evolution, humans have only relatively recently begun consuming alcohol in relatively large quantities in beverages, far too recently to expect that these enzyme systems evolved in response to humans drinking beer or wine. Rather, these enzymes are likely present to metabolize the products of microbial fermentation reactions that occur in our own intestinal tract (bacteria in our gut produce ethanol, for example) and the alcohol compounds present in small doses in foods we eat (orange juice, for example, contains small amounts of methanol, among other alcohols).

Metabolism of methanol
A consequence of having a common enzyme system for metabolizing multiple alcohols is that not all are converted to safer molecules. Let’s look at the oxidation of methanol.
ADH converts methanol (structure shown below) into which of the following compounds?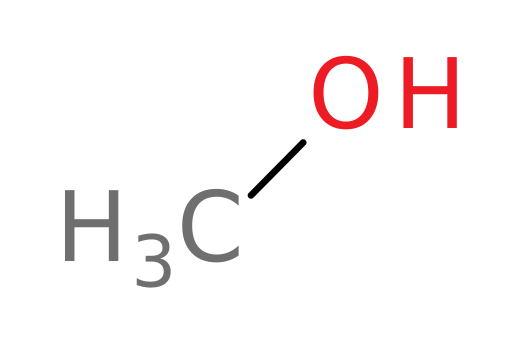
| A. |
| B. |
| C. |
| D. |
| E. |
| F. |
Formaldehyde is further metabolized by ALDH into which product?
| A. |
| B. |
| C. |
| D. |

Metabolism of ethylene glycol
Let’s see what happens when someone ingests normal antifreeze containing ethylene glycol to find out why the ER doctor and nurse in the original case introduction were so concerned about the young girl.
The main component of antifreeze is ethylene glycol (structure shown below).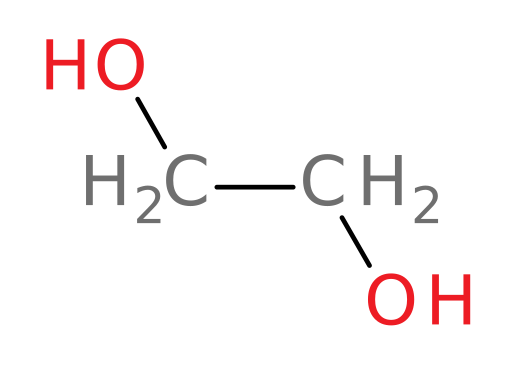
Which of the following would be the product after ethylene glycol is completely metabolized by ADH? (Note: In this case, because the molecule is a polyalcohol with two hydroxyl groups, it will require two full cycles of enzyme- catalyzed oxidation until ADH is completely finished.)
| A. |
| B. |
| C. |
| D. |
| E. |
After further oxidation by ALDH, the final product in ethylene glycol metabolism corresponds to which structure? (Hint: Again, it will require two rounds of enzyme-catalyzed oxidation.)
| A. |
| B. |
| C. |
| D. |
| E. |

ADH inhibitors as life-saving drugs
Why might Fomepizole, an ADH inhibitor, save someone who has ingested antifreeze containing ethylene glycol? (Hint: see pages 234-242 in Section 8.5 of Stryer Biochemistry, 8th ed., for more information about enzyme inhibitors and review the reactions involved in ethylene glycol metabolism above.)
| A. |
| B. |
| C. |
| D. |
| E. |
The following structure is that of fomepizole. This compound acts as a competitive inhibitor to ADH, meaning that it: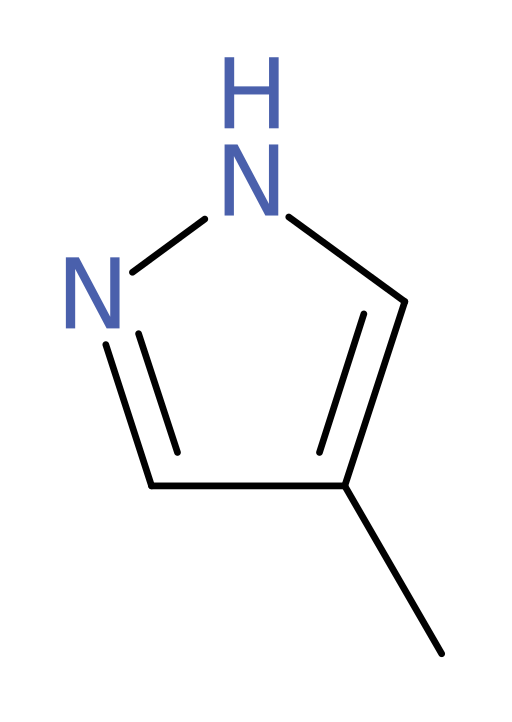
| A. |
| B. |
| C. |
| D. |
| E. |
ADH requires the presence of a nicotinamide adenine dinucleotide (NAD+) molecule in order to catalyze the oxidation of an alcohol. Because NAD+ is converted to NADH and released, it is actually a cosubstrate of the reaction (and NADH is a coproduct). Also, like many enzymes, ADH requires a metal cation for catalysis, in this case Zn2+. Zn2+ is not altered and is not tightly bound to ADH. What term best describes the role of Zn2+ in the ADH reaction? (Hint: Review the information about cofactors, coenzymes and prosthetic groups on pages 216-218 and Table 8.2 of Stryer Biochemistry, 8th ed. before attempting this question!)
| A. |
| B. |
| C. |
| D. |
Methanol and ethylene glycol are very dangerous, even in small doses. Before drugs like fomepizole were created, and still whenever they are not available, an alternative treatment for methanol poisoning is to administer ethanol. Review the structures of ethanol and methanol and recall the substrate specificities of ADH and ALDH. Now examine the following options. How might ethanol help someone who has recently ingested methanol?
| A. |
| B. |
| C. |
| D. |
| E. |
| F. |
| G. |
If a person has ingested methanol, will administering ethanol ultimately slow the rate at which formaldehyde is converted into formic acid?
| A. |
| B. |
| C. |
| D. |
| E. |

Metabolism of propylene glycol
We now examine our last alcohol in this case: propylene glycol. Propylene glycol (structure shown below) is very similar in structure to ethylene glycol and so it is also often used as a main component in some antifreeze products.
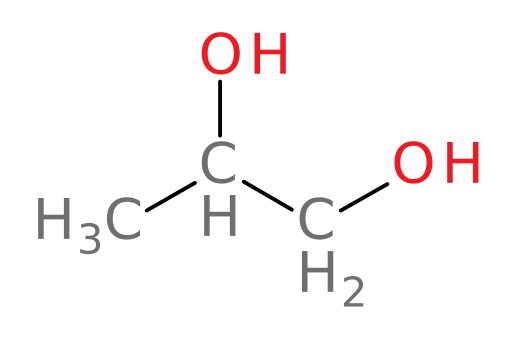
Let’s now examine how ADH and ALDH oxidize propylene glycol in the liver.
ADH metabolizes propylene glycol into which of the following compounds?
| A. |
| B. |
| C. |
| D. |
| E. |
After further oxidation by ALDH, the final product in propylene glycol metabolism corresponds to which structure?
| A. |
| B. |
| C. |
| D. |
| E. |
Do you know the name of this final product in propylene glycol metabolism by ADH and ALDH? If you keep studying biochemistry you will! It’s a very important molecule for all living organisms: pyruvic acid (ionized form: pyruvate), a central intermediate of multiple biochemical pathways in human metabolism. Like ethanol, once fully metabolized, it provides nutritional calories and has minimal toxic effects.
Because propylene glycol has similar physical properties to ethylene glycol but is less toxic, it has been used as the main component of ”pet-safe antifreeze.” In fact, you may recall that propylene glycol is actually the least toxic alcohol discussed in this case study, and so you may also find propylene glycol listed as an additive to food and cosmetic products. The only difference in structure between propylene glycol and ethylene glycol is that propylene glycol has one additional methyl group. This additional methyl group makes little difference to the toxicity of the alcohols themselves, but has a huge impact on the toxicity of the final molecule that is produced by the total oxidation by ADH and ALDH in the liver: highly dangerous oxalic acid vs. pyruvic acid, a central metabolite that makes propylene glycol basically a food substance in small quantities!
Match each alcohol to its corresponding final metabolic product following the oxidation reactions catalyzed by ADH and ALDH:
Ethylene glycol Methanol Isopropanol Propylene glycol Ethanol | Oxalic acid (crystallizes with Ca2+) Acetic acid (vinegar) Acetone (organic solvent) Formic acid (ant sting venom) Pyruvic acid (central metabolite) |
| Correct Matches: | |
The dose makes the poison: Ethanol is highly toxic in the quantities in which it is often consumed
Although it is the second least toxic of the five alcohols discussed in this case, ethanol is not a safe substance to ingest in large quantities. One serious problem with alcohol consumption is how the quantity is conveyed to the buyer. For example, a certain canned alcoholic beverage on the market is sold in 23.5 fluid ounce (695 mL) cans and is 12% (v/v) ethanol. These cans, although officially labeled as containing multiple servings, are considered “single servings” by many young people, who can easily overdose on ethanol as a result of drinking two cans, for example. Two cans may not sound like a lot, but . . .
Calculate the total volume of pure ethanol (in mL) in just one can (23.5 fluid ounces, 695 mL, 12% (v/v) ethanol):
| A. |
| B. |
| C. |
| D. |
To put this number in perspective, calculate the total volume (in milliliters) of ethanol in a standard 750 mL bottle of liquor (whisky, vodka, rum, etc.), which is typically 40% (v/v) ethanol, and in a typical 12 fluid ounce (354.8 mL) can of light beer, which is 4% (v/v) ethanol respectively.
| A. |
| B. |
| C. |
| D. |
Now compare these volumes using percentages. In other words, the total volume of ethanol from the 23.5-ounce beverage in the earlier question is what percentage of the total volume of ethanol contained in a typical bottle of liquor and a can of light beer respectively?
| A. |
| B. |
| C. |
| D. |

Congratulations on completing this Case Study!
A final note and plea from the author
We hope that you have enjoyed learning about how having promiscuous enzymes (with low substrate specificity) results in the diverse physiological consequences of alcohol metabolism. We also hope you noticed that the ethanol found in that single 23.5-ounce beverage is approximately equivalent to drinking six 12-ounce cans of beer or more than a quarter of a bottle of liquor. Clearly this is a large quantity of alcohol, so large that two cans (equivalent to 12 beers or half a bottle of whiskey) is likely to be a lethal dose for many people! Can you think of any other commonly found food product that when consumed in two “apparent servings” is lethal to many people without any explicit label to warn the consumer? Please be cautious if you chose to drink ethanol. It is not only a beverage but also a lethal poison at easily attainable levels of consumption. Worst of all, because it inhibits your sense of caution and impairs judgment, the more you consume the less aware of, and concerned about, these dangers you will become. Finally, no one can estimate your own body’s ability to tolerate or metabolize alcohol, so it is extremely difficult to estimate safe levels of consumption in yourself or others. Many college students experience alcohol poisoning, often resulting in death, at campuses around the world. Imagine if instead of the poison called ethanol, you were consuming rat poison for fun. Do you know exactly how much rat poison you or your friends can tolerate without becoming sick? Would you encourage a friend to see how much he can consume or how fast she can consume it? Please consider these issues as you encounter alcohol in the future and help to keep yourself, your friends, and others around you safe. This author would like to see you continue to be happy and healthy biochemistry students with bright futures! Best wishes and be well.
-JH
Topic Prerequisites: This case does not require specific course coverage prior to assignment and therefore may be assigned at any time in a typical biochemistry course, although the beginning of Chapter 8 of Stryer 8E is likely the most appropriate.
Overview
The case reviews various concepts of enzyme function in the context of alcohol metabolism. Specifically, it reviews how the lack of substrate specificity in the enzymes primarily responsible for alcohol metabolism results in wildly different toxicities for various alcohols found in daily life. Students review multiple topics presented in Chapter 8 of Stryer 8E. In addition, students also review oxidation states and recall common reactions of organic functional groups (in this case, enzyme-catalyzed reactions). Finally, the case foreshadows future topics in metabolism and educates students about some of the perils of ethanol consumption (both caloric intake and acute toxicity).
Students may work through the online assessment individually or in groups, referring to their text. All assessment questions are automatically scored.
Learning Objectives
This case is intended for remediating or extending student capabilities in these difficult topics:
1. Real-world application of the study of human enzyme-catalyzed reactions. Students will:
2. Review of various aspects of enzyme function, which include:
3. Practice of critical thinking skills involving data. Students will:
Suggested implementation
Below we describe two options for course implementation. The Hybrid Online/In-class approach is recommended. Time required for students to complete the online case will vary by group or student. The case study can be started and stopped, and so it is recommended that students be given a window of 2 to 3 days in which to complete the assignment.
Hybrid: Online/In-class: (recommended approach; ~20 minutes of class time expected)
1. Share the case study link with your students to allow them to work online outside of class in groups or alone. Assign the case study to be due before your next class meeting. Students should be instructed to bring copies of notes and/or printed answers to assessment questions to the following class period.
2. Review the online answers before the following class to spot difficult areas for students.
3. Lead students in a discussion in pairs, groups, or as a class (depending upon class size and instructor preference) to address unresolved difficulties (~20 minutes in-class time).
4. We recommend using the supplied assessment questions on exams or as homework assignments to reinforce any difficult concepts. Please see document “Exam Questions for Toxic Alcohols—Enzyme Function.”
Online only approach: (minimal in-class time required)
1. Share the case study link with your students to allow them to work online, outside of class in groups, or alone. Assign the case study to be due before your next class meeting.
2. Review the online answers to spot difficult areas for students.
3. Mention or remediate tough points during a portion of lecture.
4. We recommend using the supplied assessment questions on exams or as homework assignments to reinforce the difficult concepts covered. Please see the document “Exam Questions for Toxic Alcohols—Enzyme Function.”
Suggestions for in-class discussions (These questions may also be used in summative assessments, e.g., exams, scored quizzes, etc.) :
You may be missing vital information needed to sufficiently explain this incident. You must complete all investigations before proceeding to the final assessment questions.
You may be missing vital information needed to sufficiently explain this incident. You must complete all investigations before proceeding to the final assessment questions.
This activity has already been completed, however feel free to review the information contained within.