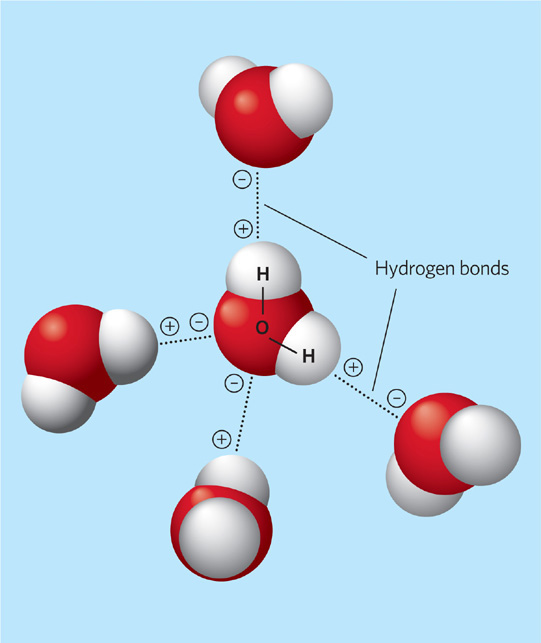
Figure 2.6 Water molecules. Because of the configuration of water molecules, they are negatively charged on the oxygen end and positively charged on the hydrogen end. The attractive forces of these opposite charges, known as hydrogen bonds, allow water molecules to be attracted to each other and to the charged ions of other compounds such as salts and sugars.