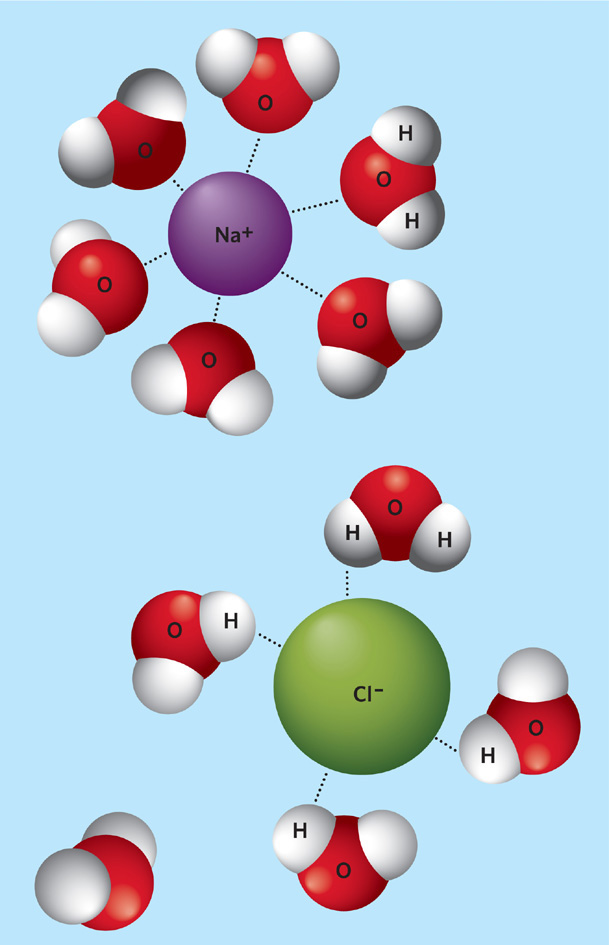
Figure 2.7 Dissolving ions in water. Because water molecules have negative and positive ends, they attract the negatively and positively charged ions, such as the sodium and chlorine ions found in sodium chloride. The forces of attraction to water molecules are stronger than the forces of attraction within the crystal, so the ions separate and become surrounded by water molecules.