Competition occurs when individuals experience limited resources
Intraspecific competition Competition among individuals of the same species.
When we study competition, we differentiate between intraspecific competition, which is competition among individuals of the same species, and interspecific competition, which is competition among individuals of different species. We considered intraspecific competition in our discussion of negative density dependence in Chapter 12, where we saw that an increase in a population’s density causes a decline in the growth rate of the population. We have discussed interspecific competition much less. Interspecific competition can cause the population of one species to decline and eventually die out. Both intraspecific and interspecific competition play substantial roles in determining the distribution and abundance of species on Earth.
Interspecific competition Competition among individuals of different species.
369
In this section, we will examine how competition for a limited resource can cause one species to outcompete another. We will explore the wide variety of resources that are available to organisms, including resources that are fixed in abundance and those that are renewable. With our understanding of resources, we will then investigate the importance of the most limiting resource in a population and examine patterns of competition between closely and distantly related species.
The Role of Resources
A resource is anything an organism consumes or uses that causes an increase in the growth rate of a population when it becomes more available. For plants, resources generally include sunlight, water, and soil nutrients such as nitrogen and phosphorus. Each is used by most plants and plays a role in the growth of plant populations. Resources for animals generally include food, water, and space. For example, animals such as mussels and barnacles spend most of their lives attached to rocks in the intertidal zone along the ocean shores (Figure 16.1). Open space is a critical resource for them because as the rocks become more crowded there is less space to grow. As a result, the growth and fecundity of adults decline, and there are few places on the rocks for offspring to settle. Similarly, many birds may compete for a limited number of nest sites or cavities, and many prey species compete for a limited number of holes and crevices in which they can hide from predators. In each of these cases, when more space becomes available, the populations can increase.

Resource Anything an organism consumes or uses that causes an increase in the growth rate of a population when it becomes more available.
Ecological factors that cannot be consumed or used are not considered resources. Temperature, for instance, plays a major role in the growth and reproduction of organisms in nature, but temperature is not a resource because it is not consumed or used. The same is true for other abiotic factors, including pH and salinity. These can affect the growth and reproduction of organisms, but because organisms do not consume or use them, they are not resources and species do not compete for them.
Renewable versus Nonrenewable Resources
Renewable resources Resources that are constantly regenerated.
We can categorize resources as either renewable or nonrenewable. Renewable resources are constantly regenerated. For example, the rodents and ants described at the beginning of the chapter compete for seeds, and every year new plants grow and renew the seed supply. Similarly, sunlight is continually generated by the Sun. In contrast, nonrenewable resources are not regenerated. For instance, space is a resource that typically has a fixed availability. If we think of the rocky intertidal habitat, there is a fixed number of rocks to which algae and animals can attach; space becomes available only when a competitor leaves or dies.
Nonrenewable resources Resources that are not regenerated.
370
Renewable resources can originate from either inside or outside the ecosystem in which the competitors live. For example, dead leaves that fall into streams from the surrounding forest serve as food for stream insects. Because these resources come from outside the system, however, competition can reduce resource abundance but cannot affect the rate of the resource supply. Moreover, resources originating from outside the system do not respond to the rate of resource consumption. Sunlight continually strikes the surface of Earth regardless of the rate at which plants and algae consume it, and the amount of local precipitation is largely independent of the rate at which plants use water.
Competitors can affect both the supply of and demand for resources that originate within the ecosystem. When competing herbivores or competing predators consume another species in the system, they reduce the supply of the species that they consumed. However, reducing the abundance of a consumed species affects its growth and reproduction, so consumption also affects the future supply of the resource. We saw an example of this with the competition between rodents and ants. When rodents were eliminated from the enclosures, the increased ant population continually ate the small seeds, and after a few years there were fewer plants that produced small seeds.
In some cases, the supply rate of a renewable resource generated within an ecosystem is only indirectly affected by competitors. For example, plants take up nitrate from the soil and use it to grow. Competing herbivores that consume the plants return large amounts of nitrogen compounds back to the soil when they defecate and after death when their bodies decompose. These nitrogen compounds are further broken down by microorganisms, which release the nitrogen as nitrate, a form plants can use. The herbivores can affect the supply rate of nitrate to the plants, but the chain of events has many links and takes such a long time that the herbivores do not have an immediate effect on the rate of future plant population size through this indirect pathway.
Leibig’s Law of the Minimum
Although consumers can reduce the abundance of both renewable and nonrenewable resources, not all resources limit consumer populations. For example, all terrestrial animals require oxygen, but an increase in the population of an animal species does not depress the concentration of oxygen in the atmosphere to the point that population growth is limited. Well before the concentration of oxygen can become limiting, some other resource, such as food supply, will decline in abundance to the point that it limits the population’s growth.
Liebig’s law of the minimum Law stating that a population increases until the supply of the most limiting resource prevents it from increasing further.
At one time, ecologists thought that populations were limited by the single resource that was most scarce relative to demand. This is known as Liebig’s law of the minimum, after Justus von Liebig, a German chemist who articulated the idea in 1840. According to Liebig’s law of the minimum, each population increases until the supply of the most limiting resource prevents it from increasing further.
The amount of a resource that limits a population’s growth depends on the resource. For example, the microscopic, glass-shelled algae known as diatoms require both silica and phosphate to grow and reproduce. When one diatom species, Cyclotella meneghiniana, is grown under different concentrations of each element, the population growth ceases whenever the concentration of silica is reduced to 0.6 micromolar (μM) or whenever the concentration of phosphate is reduced below 0.2 μM. According to Liebig’s law of the minimum, whichever resource reaches its limiting value first will be the resource that regulates the growth of the diatom population.
If we know the minimum amount of a resource that is required for populations to grow, we should be able to predict which species is the best competitor for the resource. An experiment with two species of diatoms, shown in Figure 16.2, demonstrates how competition for silica reduces the availability of silica and affects the outcome of competition. When the two species are raised separately, Asterionella formosa and Synedra ulna both experience rapid population growth followed by a plateau as they reach their carrying capacity, shown in Figure 16.2a. When Asterionella formosa reaches its carrying capacity, it drives down the abundance of silica to 1 μM. However, when Synedra ulna reaches its carrying capacity, it drives down the abundance of silica to 0.4 μM, which is not enough silica to support the other diatom’s population. As a result, Synedra ulna should outcompete Asterionella formosa. When a researcher put both species together, that is exactly what happened. As illustrated in Figure 16.2b, the two species drove the abundance of silica down to a level that allowed Synedra ulna to persist but caused Asterionella formosa to decline to extinction. The outcome between these two diatoms is one that is commonly observed in nature: when two species compete for a single limiting resource, the species that persists is the one that can drive down the abundance to the lowest level.
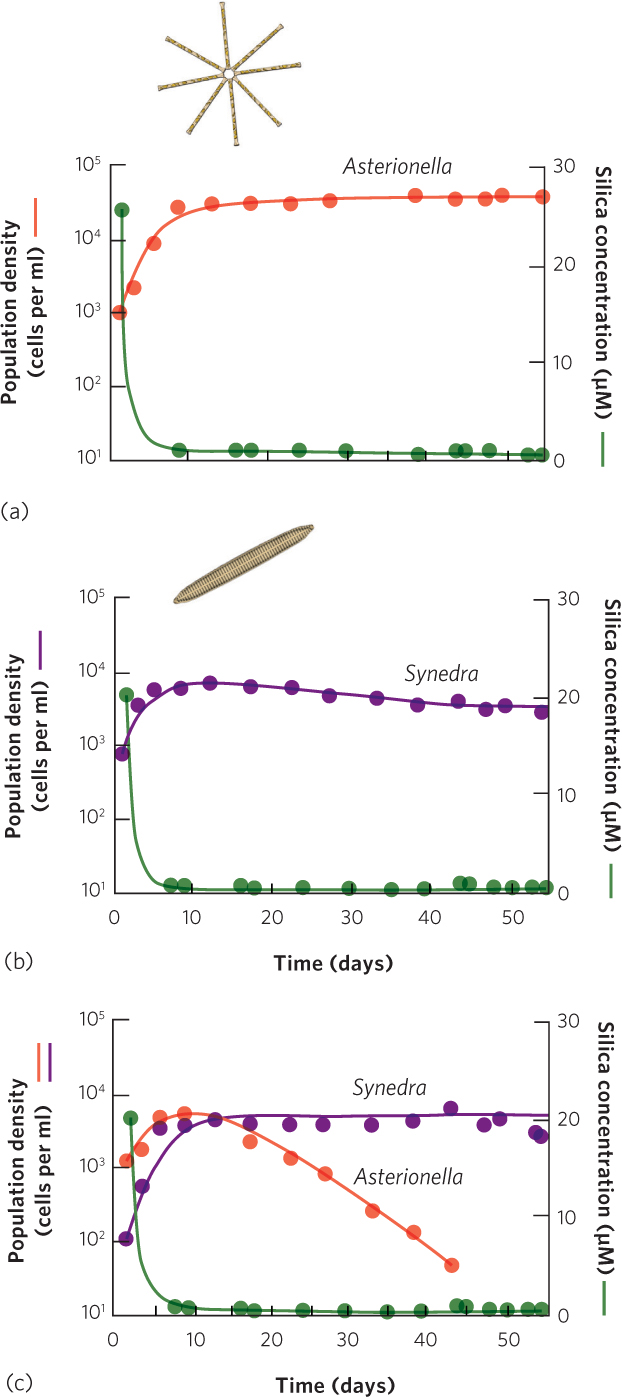
371
Interactions Among Resources
Leibig’s law of the minimum assumes that each resource has an independent effect on the growth of a population. In other words, it assumes that if a given resource limits the growth of individuals and populations, increasing the availability of other resources will not improve this growth. However, this is not always the case, as seen in research on small balsam (Impatiens parviflora), a plant that is common in English woodlands. Researchers wanted to know if this plant would respond independently to two different resources: fertilizer and light. They sowed seeds of the plants in pots filled with soil. Control pots received only water while fertilized pots received a solution containing both water and fertilizer. These two groups of pots were then grown under one of four different light intensities for 5 weeks. As you can see in Figure 16.3, plants grown in low-nutrient soil experienced only a small increase in growth when the amount of light was increased. Similarly, plants grown at a low light intensity experienced a small growth increase when soil nutrients were increased. However, plants grown under high soil fertility and high light intensity experienced an increase in growth that was much larger than the sum of the separate effects of fertility and light intensity. This means that an increase in one resource can have a much larger effect on a population when there is a simultaneous increase in a second resource.
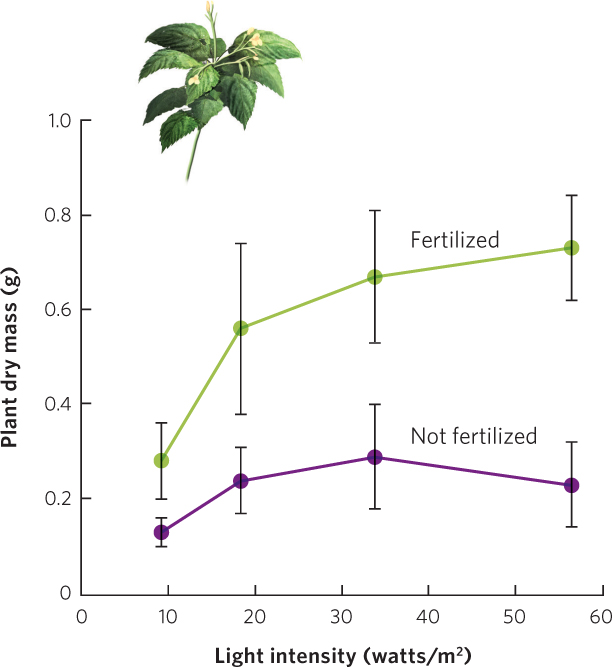
372
The Competitive Exclusion Principle
A classic study of competition between species was conducted by Russian biologist Georgyi Gause in the 1930s. As you may recall from Chapter 12, Gause conducted laboratory experiments that explored how populations of protists in the genus Paramecium grew when living alone or together with limited resources. He began by growing two species—P. aurelia and P. caudatum—separately in test tubes. The test tubes contained a fixed amount of food—the bacteria Bacillus pyocyaneus. When grown separately, the population of each paramecium species initially experienced rapid growth and then began to plateau as it reached its carrying capacity. You can see these data in the first two graphs of Figure 16.4. However, when the two species were grown together in the test tube, Gause observed a different outcome. As you can see in Figure 16.4c, the population of P. aurelia persisted in the test tube but the population of P. caudatum declined to very low levels by the time the experiment ended. In short, P. aurelia was the superior competitor, presumably by driving down the abundance of the bacteria to such a low point that P. caudatum could not persist.
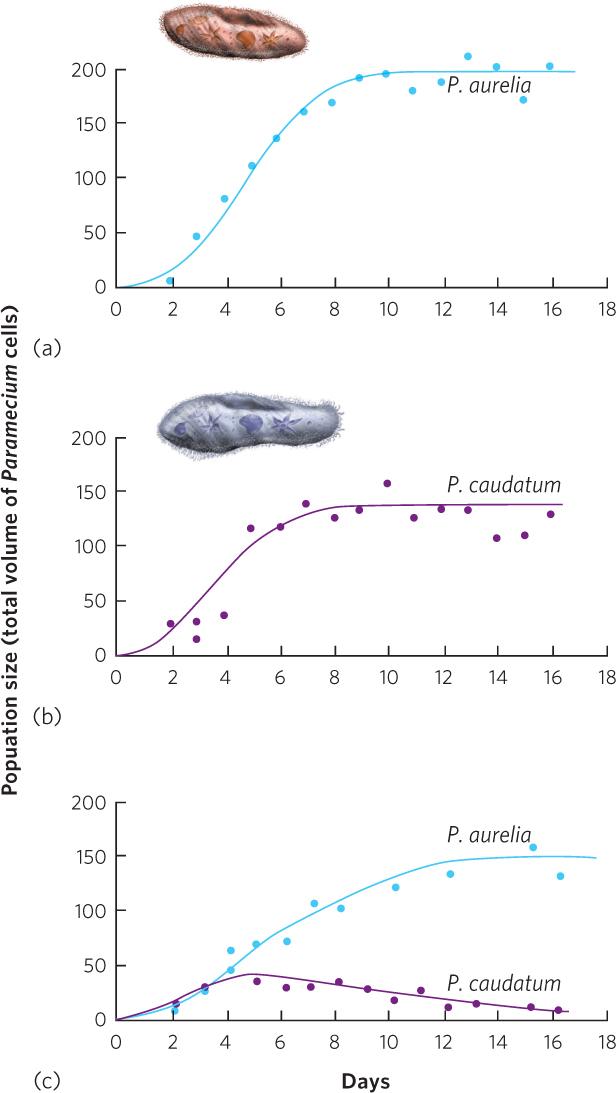
Competitive exclusion principle The principle that two species cannot coexist indefinitely when they are both limited by the same resource.
Similar competition experiments have been conducted hundreds of times using a wide variety of organisms. These experiments frequently produce the same result: one species persists and the other dies out. This common pattern led to the development of the competitive exclusion principle, which states that two species cannot coexist indefinitely when they are both limited by the same resource. Researchers commonly find that when two species are limited by the same resource, one species either is better at obtaining the resource or is better able to survive when the resource is scarce.
Competition Among Closely Related Species
As we saw in the case of ants and rodents, competition can occur between both closely and distantly related organisms. Charles Darwin believed that competition is most intense between closely related species because, he reasoned, they possess similar traits, probably consume similar resources, and, therefore, have the potential to compete strongly.
After Darwin, naturalists observed that closely related species living in the same region often grow in different habitats. They hypothesized that because these species would compete strongly for the same resources, natural selection would favor differences in habitat use. These differences in habitat use would allow each species to have a competitive advantage within its preferred habitat and to have a competitive disadvantage in habitats preferred by closely related species.
373
The first experimental test of this hypothesis was conducted in 1917 by the British botanist Arthur Tansley, who worked with two species of small perennial plants known as bedstraw. Heath bedstraw (Galium saxatile) typically lives on acidic soils whereas white bedstraw (G. sylvestre) typically lives on alkaline soils. To determine whether each species competed best under the conditions where it naturally occurred, Tansley and his colleagues planted them separately and together in deep boxes containing either acidic or alkaline soils, as illustrated in Figure 16.5. Because the boxes were located at a single site—the Botanic Garden in Cambridge, England—any differences in growth would be due to the types of soils in which the bedstraw was planted.

When planted separately, each species germinated and grew in both types of soil. However, each species presented more vigorous germination and growth in the soil type characteristic of its natural habitat. When grown together on alkaline soils, the white bedstraw overgrew and shaded the heath bedstraw. When grown together on acidic soils, heath bedstraw outcompeted the white bedstraw. Tansley concluded that although both species are capable of living in both soil types when they are grown separately, intense competition from a closely related species restricts their distribution in nature to the soil type that gives them a competitive advantage. Hundreds of studies inspired by the work of Tansley have supported his finding that many closely related species are intense competitors and are therefore distributed among different habitats in ways that reduce overlap, and thus competition, with each other.
Competition Among Distantly Related Species
While competition can be quite intense among closely related species, it can also be intense among distantly related species that consume a common resource. As we saw in Figure 16.1, open space on rocks in the intertidal biome is a resource used by numerous distantly related species such as barnacles, mussels, algae, sponges, and others, all of which compete intensely for this limited space. As we will see later in this chapter, the outcome of this competition among so many competitors depends on their abilities to compete as well as their abilities to tolerate different abiotic conditions and predation.
Another example of competition among different species occurs among animals that eat krill (Euphausia superba). Krill are shrimp-like crustaceans that live in the oceans surrounding Antarctica, and are consumed by virtually every type of large marine animal, including fish, squid, penguins, seals, and whales. Commercial exploitation of whales in the Southern Hemisphere has caused a decline in whale populations while penguin and seal populations have increased. This suggests that reducing the number of whales has eased competition for krill and allowed penguin and seal populations to grow.
Intense competition can also occur among distantly related species in terrestrial ecosystems. On the forest floor, spiders, ground beetles, salamanders, and birds all consume the invertebrates that live in the leaf litter. In desert ecosystems, birds and lizards eat many of the same insect species. As we discussed at the beginning of the chapter, desert ants and rodents compete for seeds. When we examine the patterns of seed sizes consumed by ants and rodents as shown in Figure 16.6, we can see that the two groups both consume a wide range of seed sizes, but the ants tend to consume more of the small seeds whereas the rodents tend to consume more of the large seeds.
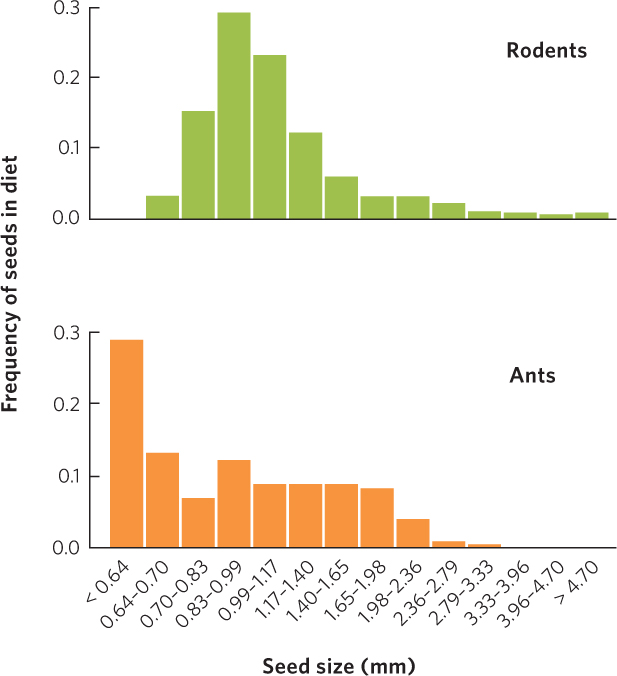
374