Mutualisms can aid in defense against enemies
As we saw with the alpheid shrimp, mutualisms can increase the ability of a species to defend itself against enemies. To obtain a defense from a mutualistic partner, an organism must provide some type of benefit in return. In this section, we will examine a number of ways that mutualisms have evolved to benefit both the species being defended and the species providing the defense.
Plant Defense
Plants are involved in a number of mutualisms that help defend them from enemies. Two well-known examples are the mutualisms between ants and acacia trees and the mutualisms between fungi and plants.
Acacia trees are found in tropical forests throughout the world; they face a variety of herbivores and numerous competitors including vines that try to wrap around the acacia’s branches. In Central America, acacia trees are commonly inhabited by ants in the genus Pseudomyrmex that constantly patrol the tree branches. The acacia trees provide large thorns with pulpy centers that ants hollow out and convert into nests. The trees also contain nectaries, which produce nectar that the ants consume (Figure 17.7a). In exchange, these ants bite and sting any herbivores—from small insects to large mammals—that attempt to consume the leaves. The ants also eliminate plants that attempt to grow near their home tree by chewing on them until they die (Figure 17.7b).
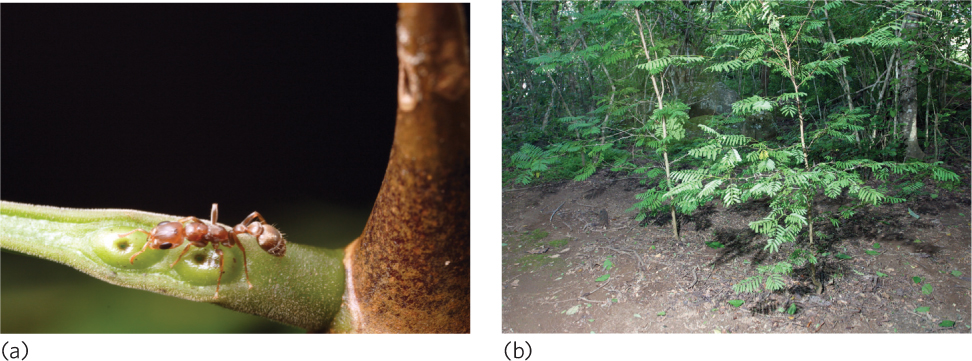
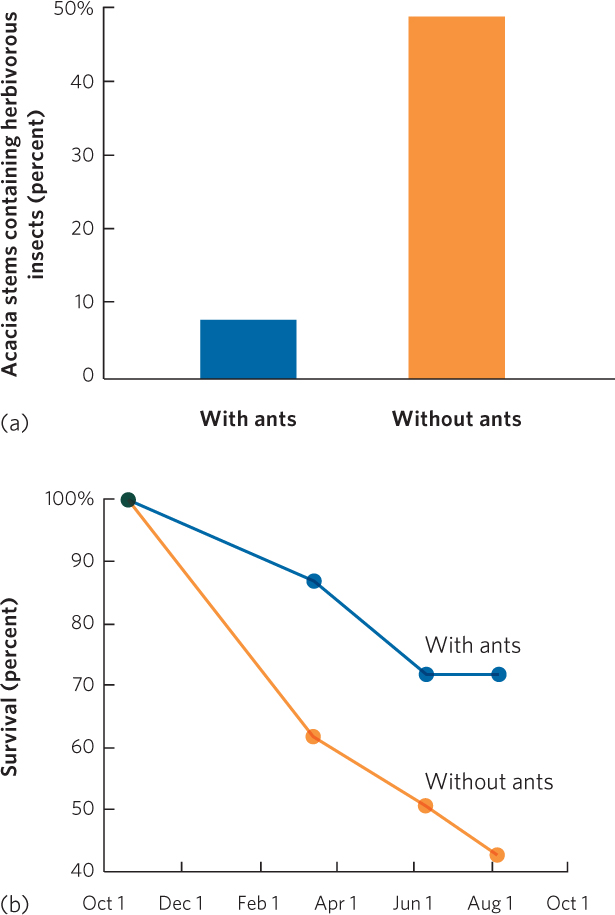
In a classic study, Dan Janzen compared acacia trees that had ants living on them and acacia trees from which he removed the ants. As you can see in Figure 17.8a, he found that trees with ants had a lower percentage of herbivorous insects than trees from which the ants had been removed. In addition, trees with ants grew to be 14 times heavier than trees without ants. When Janzen monitored the trees over a 10-month period, he found that trees with ants had much higher survival rates than trees without ants, as shown in Figure 17.8b. In short, Janzen demonstrated that the ants are critical to the survival and growth of the acacia trees.
398
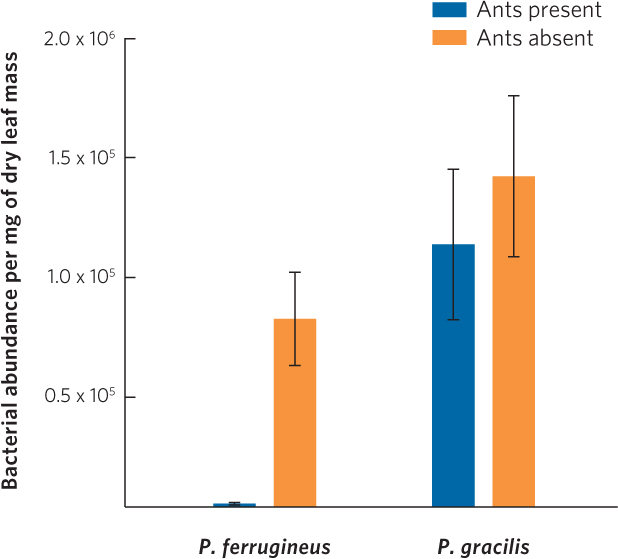
More recently, researchers have discovered that the ant-acacia mutualism has additional benefits. In 2010, researchers reported that the chemicals in the nectaries contain numerous proteins with antibacterial properties. To determine if ants helped to distribute these chemicals on the leaves, the researchers used two sets of trees: one group had the ants present and the other group had the ants removed for 2 weeks. Researchers also examined the effect of one ant species that acted as a mutualist (P. ferrugineus) and another ant species that did not (P. gracilis). You can view the results of the experiment in Figure 17.9. When P. ferrugineus was present, the plant leaves were nearly free of bacteria, but when P. gracilis was present, there was no significant reduction in bacteria. The researchers do not yet know how the P. ferrugineus causes a reduction in leaf bacteria, but it seems likely that P. ferrugineus is distributing some of the antibacterial nectar to the leaves whereas P. gracilis does not. These results demonstrated that ants in a mutualistic relationship with trees not only defend the tree against herbivores and competitors but appear to also defend the tree against bacteria that act as plant pathogens.
Endophytic fungi Fungi that live inside a plant’s tissues.
Some plants can defend themselves from herbivores through mutualisms with fungi known as endophytic fungi that live within the plant’s tissues. These fungi produce chemicals that can repel insect herbivores and also provide drought resistance by increasing the concentration of minerals in plant tissues, which increases the plant’s ability to absorb and retain water from the soil. In exchange, the plant supplies the fungi with the products of photosynthesis. While endophytic fungi can be beneficial to plants, some of the chemicals they produce can be quite harmful to herbivores on which humans depend. For example, when the grass known as tall fescue (Festuca arundinacea) contains endophytic fungi, the fungi produce chemicals that are highly toxic to cattle, sheep, goats, and horses. Researchers are currently working to identify alternative strains of fungi that can offer defense against insect herbivores and drought resistance without being toxic to livestock.
Animal Defense
Animals also participate in mutualisms that aid their defense. An excellent example occurs in a group of fish known as cleaner wrasse. These tiny fish spend their life consuming ectoparasites that are attached to other, much larger fish (Figure 17.10). As the cleaner wrasse approaches, the larger fish opens its mouth and flares its gills to permit access to the many parasites that are attached to its body. The number of parasites removed can be substantial; a single cleaner wrasse can consume more than 1,200 parasites per day. The tiny cleaner wrasse benefits from having a large source of food and the larger fish benefit by having fewer parasites.

399
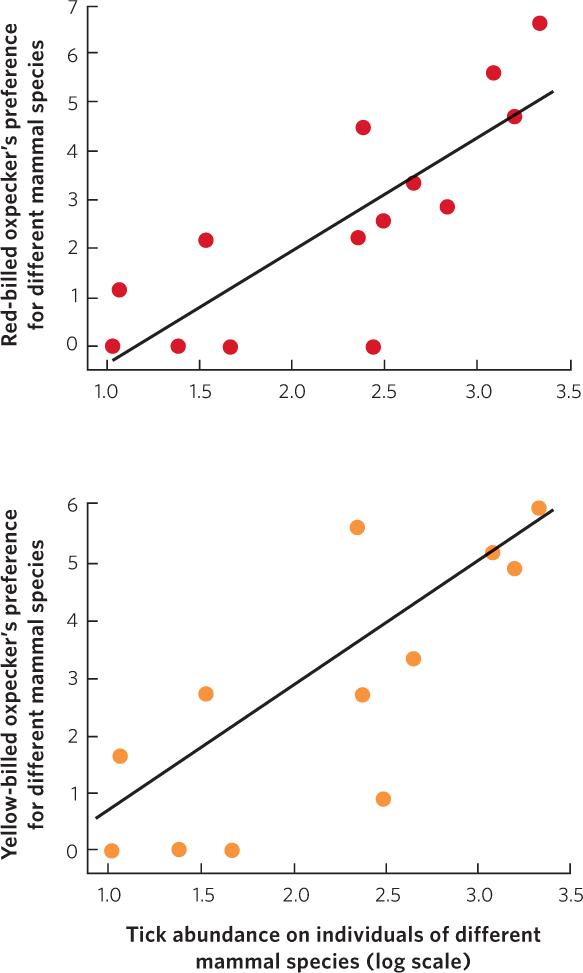
A similar situation exists for large terrestrial animals in Africa. Two species of birds, the red-billed oxpecker (Buphagus erythrorhynchus) and the yellow-billed oxpecker (B. africanus), perch on the backs of grazing animals such as rhinos and antelopes (Figure 17.11). While perched on the backs of the grazing animals, the birds consume ticks that are attached to the mammals. However, because the birds also peck at the wounds caused by the ticks, scientists wondered whether the birds were mutualists or parasites. If oxpeckers act primarily as mutualists, their preferences for certain species of grazing mammals should be related to the number of ticks carried by each species. Alternatively, if the birds act primarily as parasites that seek to peck at mammal flesh, they should prefer mammals with thinner hides, which their beaks can penetrate more easily. In a 2011 study, researchers examined these relationships in both species of oxpeckers using up to 15 species of grazing mammals in Africa. The researchers quantified the oxpecker preferences for different species of mammals by observing grazing mammals of several species and dividing the number of oxpeckers on a given species by the total number of species grazing. Then they quantified the abundance of ticks on an individual of each mammal species. Although they found no relationship between the species preferences of oxpeckers and the thickness of the animals’ hides, they found positive correlations between the species preferences of oxpeckers and tick abundance, as you can see in Figure 17.12. These results suggest that the birds are acting primarily as mutualists whose preferences are geared toward feeding on ticks.

red-billed oxpecker in Kruger National Park in South Africa, remove ticks from a variety of grazing mammals, such as this impala (Aepyceros melampus).
400