Chapter 21
Movement of Elements in Ecosystems
490

491
CHAPTER CONCEPTS
- The hydrologic cycle moves many elements through ecosystems.
- The carbon cycle is closely tied to the movement of energy.
- Nitrogen cycles through ecosystems in many different forms.
- The phosphorus cycle moves between land and water.
- In terrestrial ecosystems, most nutrients regenerate in the soil.
- In aquatic ecosystems, most nutrients regenerate in the sediments.
Living in a Dead Zone
Each summer, as the Mississippi River flows into the Gulf of Mexico, an area develops where animals can’t survive. While fish, crawfish, and crabs remain abundant in other parts of the Gulf, the summer algal bloom in this area makes it uninhabitable. In many cases, the rapidly increasing populations of algae contain green pigments that turn the water green. When the algae contain red pigments, the algal bloom is called a red tide.
Algal blooms can have both direct and indirect effects on aquatic organisms. A direct effect occurs when the species of algae or cyanobacteria that bloom produce toxins. At high algal densities, these toxins can accumulate to concentrations that impair the survival, growth, and reproduction of other species living in the area.
“The resulting dead zone in the Gulf of Mexico can cover more than 22,000 km2 during the summer—an area the size of New Jersey.”
An indirect effect of algal blooms occurs after the algae, also known as phytoplankton, bloom and die. While live algae produce oxygen during photosynthesis, zooplankton and bacteria that consume the enormous biomass of dead algae use large amounts of oxygen. This leads to a dramatic reduction in oxygen in the water and causes many of the animals in the water to die from oxygen deprivation. Aquatic ecosystems that experience algal blooms and large animal die offs are called dead zones.
What causes the large algal blooms? Researchers have discovered that many rivers carry large amounts of nutrients such as nitrogen and phosphorus that come from fertilizers that run off lawns and agricultural fields when it rains. This water runoff enters streams and rivers that join before emptying into the ocean. Other sources of nutrients include wastewater with a range of components including detergents that contain phosphorus and sewage that is released from wastewater treatment systems when they are overwhelmed by large rain events. The nutrients that rivers dump into the ocean allow rapid algal growth, which causes an algal bloom.
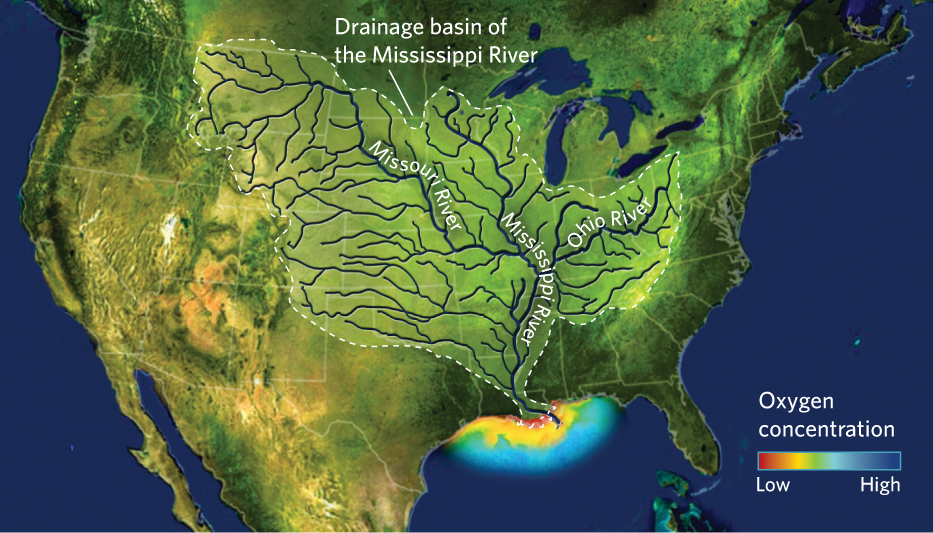
The Mississippi River drains 41 percent of the contiguous United States, so it carries nutrients gathered from a very large area. The resulting dead zone in the Gulf of Mexico can cover more than 22,000 km2 during the summer—an area the size of New Jersey. As autumn weather comes, fewer nutrients enter the Mississippi River and the temperatures in the Gulf of Mexico become cooler, conditions that make it more difficult for algal populations to increase. As a result, the dead zone disappears each winter.
Human activities cause most dead zones, although some do have natural causes. The abundance of dead zones around the world is growing rapidly. In the 1910s, only four dead zones were known to exist. This number increased to 49 dead zones in the 1960s and 87 in the 1980s. The number of dead zones rose to 305 in 1995 and increased further to 405 by 2008, the most recent date for which there is an estimate. For example, a large dead zone occurs in the Chesapeake Bay on the east coast of North America, where up to 40 percent of the bay can become hypoxic, meaning the water is low in oxygen. Similarly, the bottom of Lake Erie becomes hypoxic each summer. Around the world, dead zones cover a total area of more than 205,000 km2.
492
The existence of dead zones illustrates why we need to understand how nutrients—including water, nitrogen, and phosphorus—move within and between ecosystems and the important role that decomposition plays in recycling these nutrients. In this chapter, we will examine the movement of nutrients and how nutrients are regenerated in terrestrial and aquatic ecosystems.
SOURCES: N. N. Rabalais, R. E. Turner, and W. J. Wiseman, Jr., Gulf of Mexico hypoxia, a.k.a. The dead zone, Annual Review of Ecology and Systematics 33 (2002): 235–263.
R. J. Diaz and R. Rosenberg, Spreading dead zones and consequences for marine ecosystems, Science 321 (2008): 926–929.
 Unlike energy—which moves through ecosystems— elements such as hydrogen, oxygen, carbon, nitrogen, and phosphorus cycle among the biotic and abiotic components of ecosystems. The movements of these elements within ecosystems are affected by chemical, physical, and biological processes. To understand the movement of these elements, which exist in a variety of chemical forms, it is helpful to think of different pools in which a given element resides, as well as the different processes that are responsible for moving an element from one pool to another. For example, two important pools for carbon are the CO2 that exists in the atmosphere and the biomass of producers that use carbon to build their tissues. In this example, the process that causes carbon to move from the atmosphere to producers is photosynthesis.
Unlike energy—which moves through ecosystems— elements such as hydrogen, oxygen, carbon, nitrogen, and phosphorus cycle among the biotic and abiotic components of ecosystems. The movements of these elements within ecosystems are affected by chemical, physical, and biological processes. To understand the movement of these elements, which exist in a variety of chemical forms, it is helpful to think of different pools in which a given element resides, as well as the different processes that are responsible for moving an element from one pool to another. For example, two important pools for carbon are the CO2 that exists in the atmosphere and the biomass of producers that use carbon to build their tissues. In this example, the process that causes carbon to move from the atmosphere to producers is photosynthesis.
Organisms contain large amounts of hydrogen, oxygen, and carbon. However, as we noted in Chapter 2, organisms also need seven major nutrients: nitrogen, phosphorus, sulfur, potassium, calcium, magnesium, and iron. Some elements are required in much smaller amounts; these include silicon, manganese, and zinc. In this chapter, we will examine the biogeochemical cycles of some of the major elements on Earth by looking at interactions among biological, geological, and chemical processes. We will also explore how human activities are currently altering these cycles in ways that have far-reaching effects on ecosystems.
493