Nitrogen cycles through ecosystems in many different forms
Nitrogen is an important component of amino acids, the building blocks of proteins, and nucleic acids, which are the building blocks of DNA. Nitrogen exists in many different forms and exhibits a complex set of pathways. In this section, we will examine the cycling of nitrogen and explore the ways that human activities have altered the nitrogen cycle.
The Nitrogen Cycle
A large pool of nitrogen gas (N2) exists in the atmosphere, where it comprises 78 percent of all atmospheric gases. Nitrogen moves through five major transformations, shown in Figure 21.6: nitrogen fixation, nitrification, assimilation, mineralization, and denitrification.
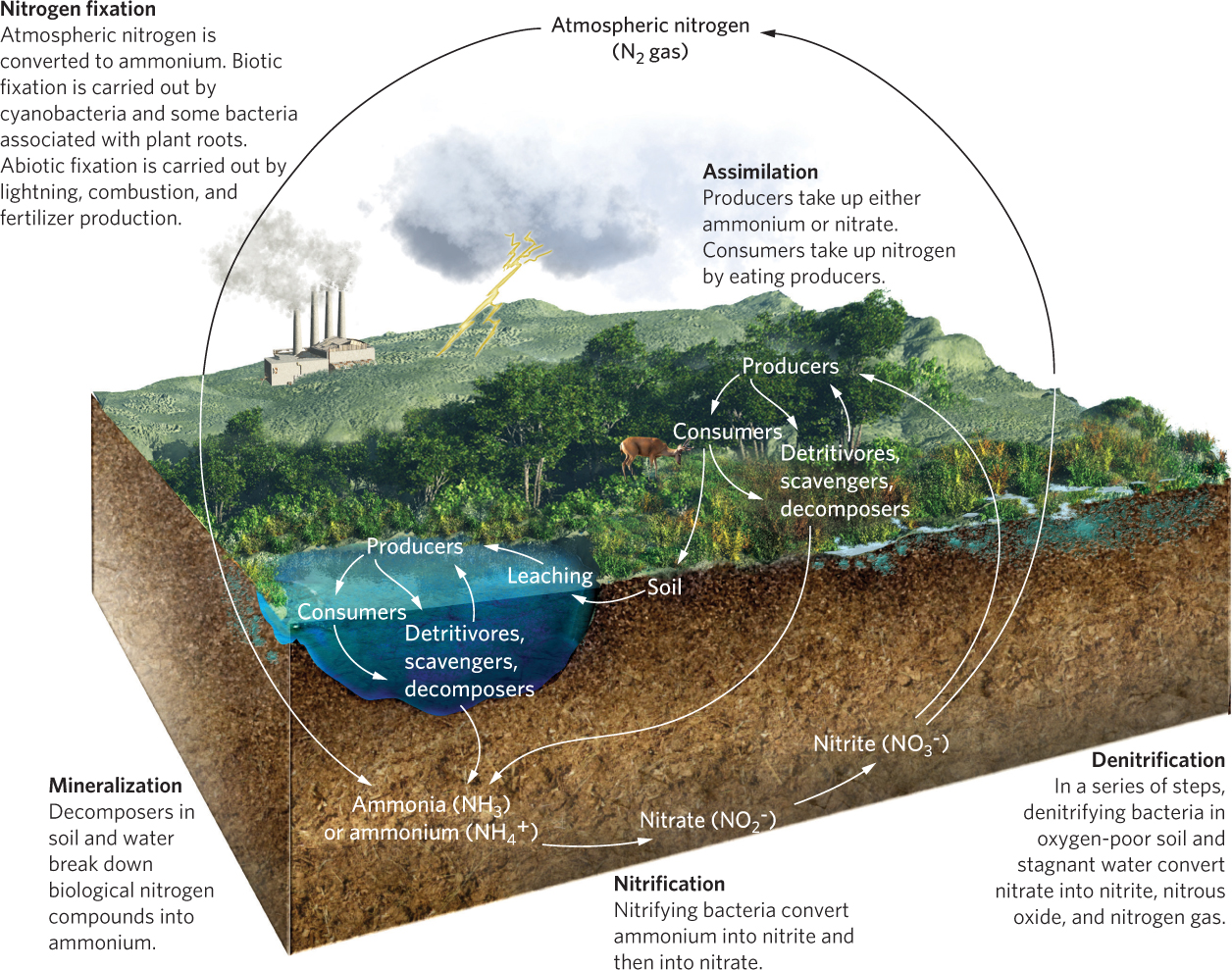
Nitrogen Fixation
Nitrogen fixation The process of converting atmospheric nitrogen into forms producers can use.
The process of converting atmospheric nitrogen into forms producers can use is known as nitrogen fixation. Nitrogen fixation converts nitrogen gas either into ammonia (NH3), which is rapidly converted to ammonium (NH4+), or into nitrate (NO3−). The compound that is formed depends on whether nitrogen fixation occurs by organisms, lightning, or the industrial production of fertilizers.
As we discussed in previous chapters, some organisms are able to convert nitrogen gas into ammonia. Nitrogen fixation occurs in some species of cyanobacteria, in some free-living species of bacteria such as Azotobacter, and in mutualistic bacteria such as Rhizobium that live in the root nodules of some legumes and other plants (see Figure 17.4). Nitrogen fixation is an important source of required nitrogen, especially for early succession plants colonizing habitats that have little available nitrogen. The process of nitrogen fixation requires a relatively high amount of energy, which nitrogen-fixing organisms obtain either by metabolizing organic matter from the environment or by acquiring carbohydrates from a mutualistic partner.
As you can see in Figure 21.6, nitrogen fixation can also occur through abiotic processes. For example, lightning provides a high amount of energy that can convert nitrogen gas into nitrate in the atmosphere. Similarly, combustion that occurs during wildfires or when fuels are burned also produces nitrates. In both cases, nitrates, which are suspended in the air after combustion, fall to the ground with precipitation.
498
The industrial production of fertilizers that improve crop productivity converts nitrogen gas to either ammonia or nitrates. Like all nitrogen fixation, this process requires a great deal of energy and is powered mostly by the combustion of fossil fuels. The manufacture of nitrogen fertilizer has developed into such a large commercial endeavor that fixation conducted by humans now exceeds the nitrogen fixation that occurs through all natural processes.
Nitrification
Another process in the nitrogen cycle is nitrification, which converts ammonium to nitrite (NO2−) and then from nitrite to nitrate (NO3−):
Nitrification The final process in the nitrogen cycle, which converts ammonium to nitrite (NO2) and then from nitrite to nitrate (NO3−).
NH4+ → NO2− → NO3−
These conversions release much of the potential energy that is contained in ammonium. Each step is carried out by specialized bacteria and archaea in the presence of oxygen. The conversion of ammonium to nitrites in terrestrial and aquatic ecosystems is carried out by Nitrosomonas and Nitrosococcus bacteria whereas the conversion of nitrites to nitrates is carried out by Nitrobacter and Nitrococcus bacteria. Although nitrites are not an important nutrient for producers, they can take them up and use them.
Assimilation and Mineralization
Mineralization The process of breaking down organic compounds into inorganic compounds.
Producers can take up nitrogen from the soil or water as either ammonium or nitrates. Once producers take up nitrogen, they incorporate it into their tissues, a process known as assimilation, which we described in Chapter 20. When primary consumers ingest producers, they can either assimilate nitrogen from the producers or excrete it as waste. The same process occurs again with secondary consumers. Animal waste, as well as the biomass of producers and consumers that eventually die, is broken down by scavengers, detritivores, and decomposers. The fungal and bacterial decomposers break down biological nitrogen compounds into ammonia. The process of breaking down organic compounds into inorganic compounds is known as mineralization.
499
Denitrification
Because nitrates produced by nitrification are quite soluble in water, they readily leach out of soils and into waterways where they settle in the sediments of wetlands, rivers, lakes, and oceans. These sediments are typically anaerobic. Under anaerobic conditions, nitrates can be transformed back into nitrites, which are then transformed into nitric oxide (NO):
NO3− → NO2− → NO
This reaction is accomplished by bacteria such as Pseudomonas denitrificans. Additional chemical reactions under anaerobic conditions in soils and water subsequently convert nitric oxide to nitrogen gas, thereby completing the nitrogen cycle:
NO → N2O → N2
Denitrification The process of converting nitrates into nitrogen gas.
This process of converting nitrates into nitrogen gas is known as denitrification.
Denitrification is necessary for breaking down organic matter in oxygen-depleted soils and sediments. However, as you can see from the above reaction, it produces nitrogen gas (N2). Since N2 cannot be taken up by producers, the denitrification process causes nitrogen to leave the waterlogged soils and aquatic ecosystems in the form of a gas.
Human Impacts on the Nitrogen Cycle
Before human activities began dramatically altering the environment, the production of usable forms of nitrogen through the process of fixation was approximately offset, on a global scale, by the loss of usable nitrogen through denitrification. However, during the last 3 centuries, and especially during the past 50 years, human activities have nearly doubled the amount of nitrogen put into terrestrial ecosystems. These activities include combustion of fossil fuels that add nitric oxide to the air, production of nitrogen fertilizers, and planting nitrogen-fixing crops.
Burning fossil fuels puts nitric oxide into the air, where it is carried to ecosystems far away. As nitric oxide enters the atmosphere, it reacts with water in the air to form nitrates, which then fall to the ground during precipitation. Because nitrogen is often a limiting nutrient, we would expect the addition of nitrates to affect a variety of ecosystems.
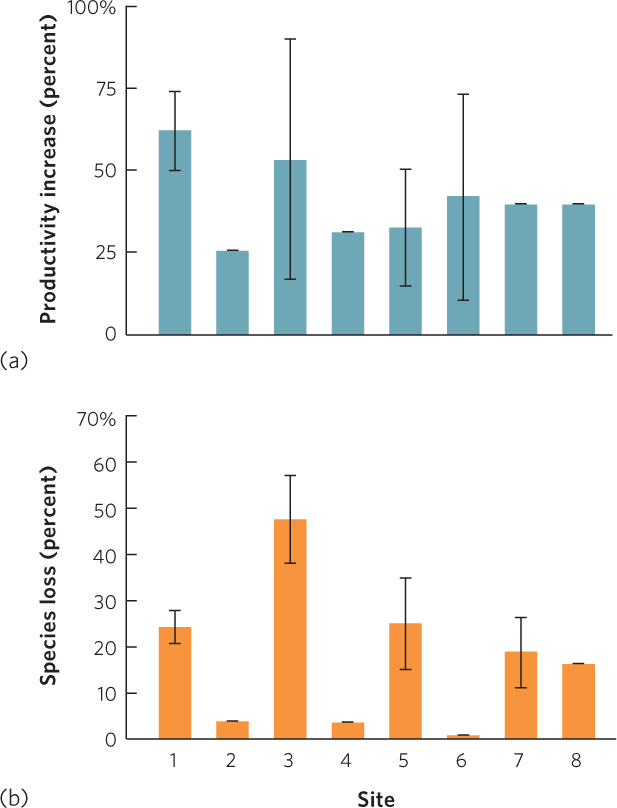
Over the years, multiple teams of researchers have investigated whether adding nitrogen to terrestrial ecosystems in North America affects productivity and species richness. These study sites, which range north to south from Alaska to Arizona and west to east from California to Michigan, were recently compiled in an effort to determine whether their results showed a general pattern. When nitrogen was added in the form of nitrates and ammonium, all sites experienced an increase in primary productivity, shown in Figure 21.7a. This confirmed that nitrogen was a limiting resource at all sites. However, the sites differed in the proportion of species that were eliminated over time, as you can see in Figure 21.7b. The researchers explored a wide range of potential causes for this variation in species loss among sites. In addition to differences in temperature and soil among the sites, they found that the sites with the largest increases in productivity experienced the largest reduction in species richness. Adding nitrogen to these communities commonly caused a few plant species to grow very large and to dominate the community. These large plants shaded the less-competitive smaller plants, which caused the smaller species to decline. These results demonstrate that the increases in nitrogen in the environment due to human activities can reduce the species diversity of ecosystems.
500