The phosphorus cycle moves between land and water
Phosphorus is a critical element for organisms because it is used in bones and scales, teeth, DNA, RNA, and ATP, a molecule involved in metabolism. As we discussed in Chapter 20, phosphorus is also a common limiting nutrient in aquatic and terrestrial ecosystems. For this reason, phosphorus is a component of most fertilizers manufactured to boost the growth of crops. In this section, we will examine the phosphorus cycle and explore how human activities have altered it in ways that affect ecosystems.
The Phosphorus Cycle
The phosphorus cycle is considerably less complicated than the nitrogen cycle. As you can see in Figure 21.8, the atmosphere is not an important component of this cycle because phosphorus does not have a gas phase; phosphorus can only enter the atmosphere in the form of dust. Unlike nitrogen, phosphorus rarely changes its chemical form; it commonly moves as a phosphate ion (PO43−). Plants take up phosphate ions from soil or water and incorporate them directly into various organic compounds. Animals eliminate excess phosphorus in their diets by excreting urine containing either phosphate ions or phosphorus compounds that are converted into phosphate ions by phosphatizing bacteria.
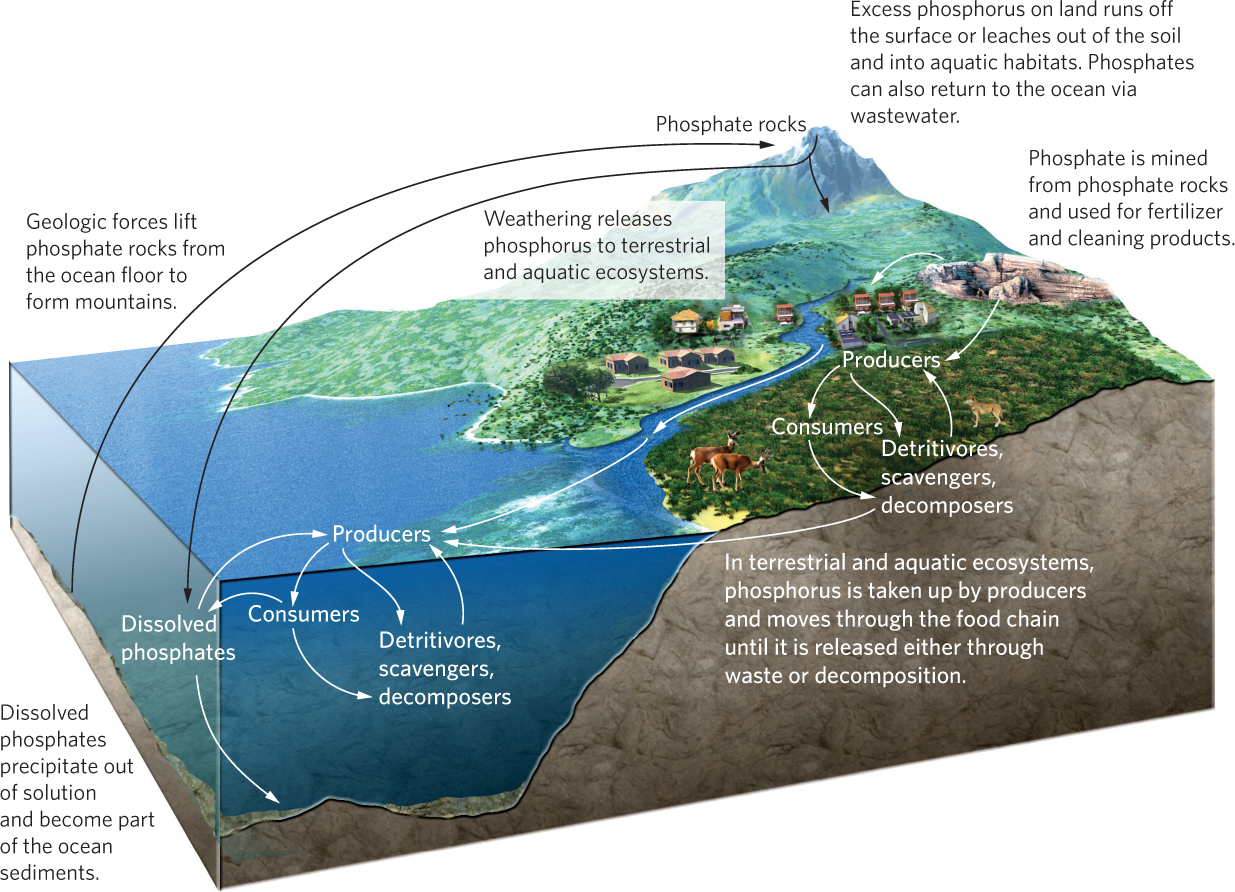
We begin our exploration of the phosphorus cycle by examining phosphate rocks, which are the major source of phosphate. As you can see in Figure 21.8, over time calcium phosphate (Ca(H2PO4)2) precipitates out of ocean water and slowly forms sedimentary rock. Later, some of this rock is uplifted by geologic forces. Exposed rocks experience weathering, which causes them to slowly release phosphate ions. Phosphate rocks are also mined for phosphate that is used in fertilizers and in a variety of detergents.
501
When phosphate ions enter terrestrial ecosystems, they can be either bound strongly to the soil or taken up by plants and passed through the terrestrial food web. Animal excretions and the decomposition of all terrestrial organisms release phosphorus back to the soil. Excess phosphorus that is not bound to the soil or taken up by plants either moves across the surface of the land during a rainstorm as runoff or leaches from the soil. When soil erosion occurs, the phosphorus that is bound to the soil is carried away with the eroding soil particles. In either case, the phosphorus can be carried to a variety of aquatic ecosystems.
When phosphate ions enter aquatic ecosystems, they are taken up by the producers and enter the food web in a manner that is similar to the terrestrial food web. In well-oxygenated waters, phosphorus readily binds with calcium and iron ions and precipitates out of the water column to become part of the sediments. Thus, marine and freshwater sediments act as a phosphorus sink by continually removing phosphorus from the water column. Under low-oxygen conditions, iron tends to combine with sulfur rather than phosphorus, so phosphorus remains more available in the water column. Over time, the phosphate that precipitates down to ocean sediments is converted into calcium phosphate rocks, and the phosphorus cycle begins again.
Human Impacts on the Phosphorus Cycle
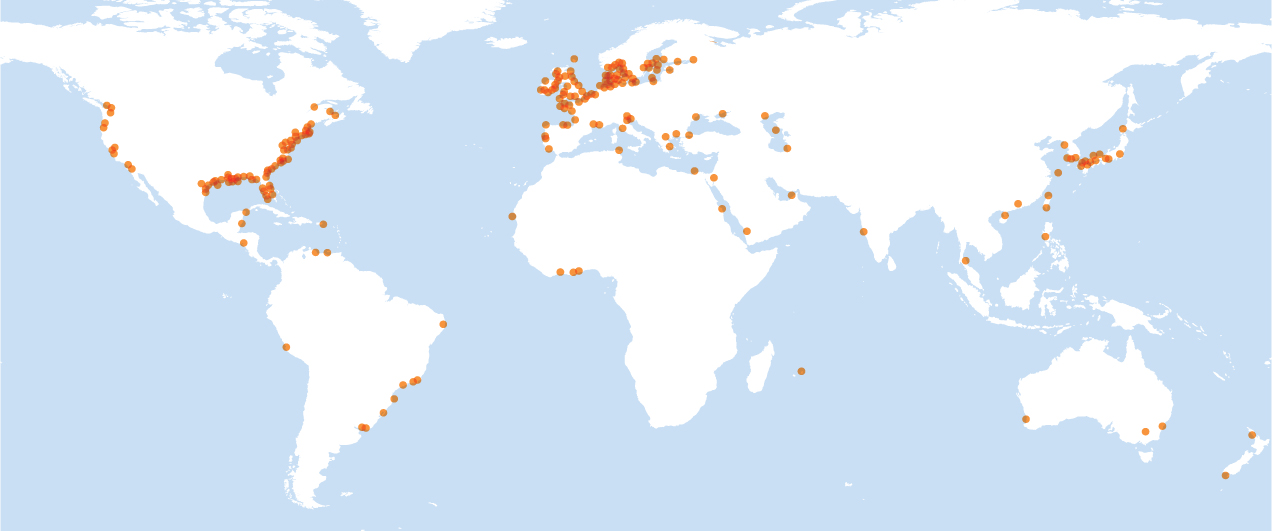
In Chapter 20 we discussed how phosphorus is commonly a limiting nutrient in terrestrial and aquatic ecosystems. Therefore, adding phosphorus to these ecosystems can have harmful effects. As we saw at the beginning of this chapter, phosphorus, sometimes in combination with excess nitrates, contributes to algal blooms that cause dead zones where rivers empty into oceans. This phenomenon happens in locations around the world, as shown in Figure 21.9. An increase in the productivity of aquatic ecosystems is called eutrophication. An increase in the productivity of aquatic ecosystems caused by human activities is called cultural eutrophication.
Eutrophication An increase in the productivity of aquatic ecosystems.
Cultural eutrophication An increase in the productivity of aquatic ecosystems caused by human activities.
From the 1940s to the 1990s, household detergents contained phosphates to improve their cleaning effectiveness. These detergents became part of the wastewater that traveled through public sewage systems, ultimately emptying into rivers, lakes, and oceans. People began to realize that these detergents significantly increased phosphorus in waterways, which contributed to eutrophication and dead zones. In 1994 the United States banned phosphates in laundry detergents after several states had already done so. In 2010, 16 states banned phosphates in dishwashing detergents.