Earth is warmed by the greenhouse effect
Solar radiation provides the vast majority of the energy that warms Earth and that organisms use. However, solar radiation alone is not sufficient to warm the planet; gases in the atmosphere also play a critical role.
The Greenhouse Effect
Solar radiation warms Earth through a series of steps, illustrated in Figure 5.1. About one-third of the solar radiation emitted toward Earth is reflected by the atmosphere—the 600-km thick layer of air that surrounds the planet—and heads back into space. The remaining solar radiation penetrates the atmosphere. Much of the high-energy radiation—including ultraviolet radiation—is absorbed in the atmosphere. The rest passes with most of the visible light through the atmosphere. When this radiation subsequently strikes clouds and the surface of Earth, a portion is reflected back into space and the rest is absorbed. As clouds and Earth’s surface absorb this radiation, they begin to warm and emit lower energy infrared radiation. The heat you feel radiating into the air if you stand on a hot asphalt road is an example of this infrared radiation.
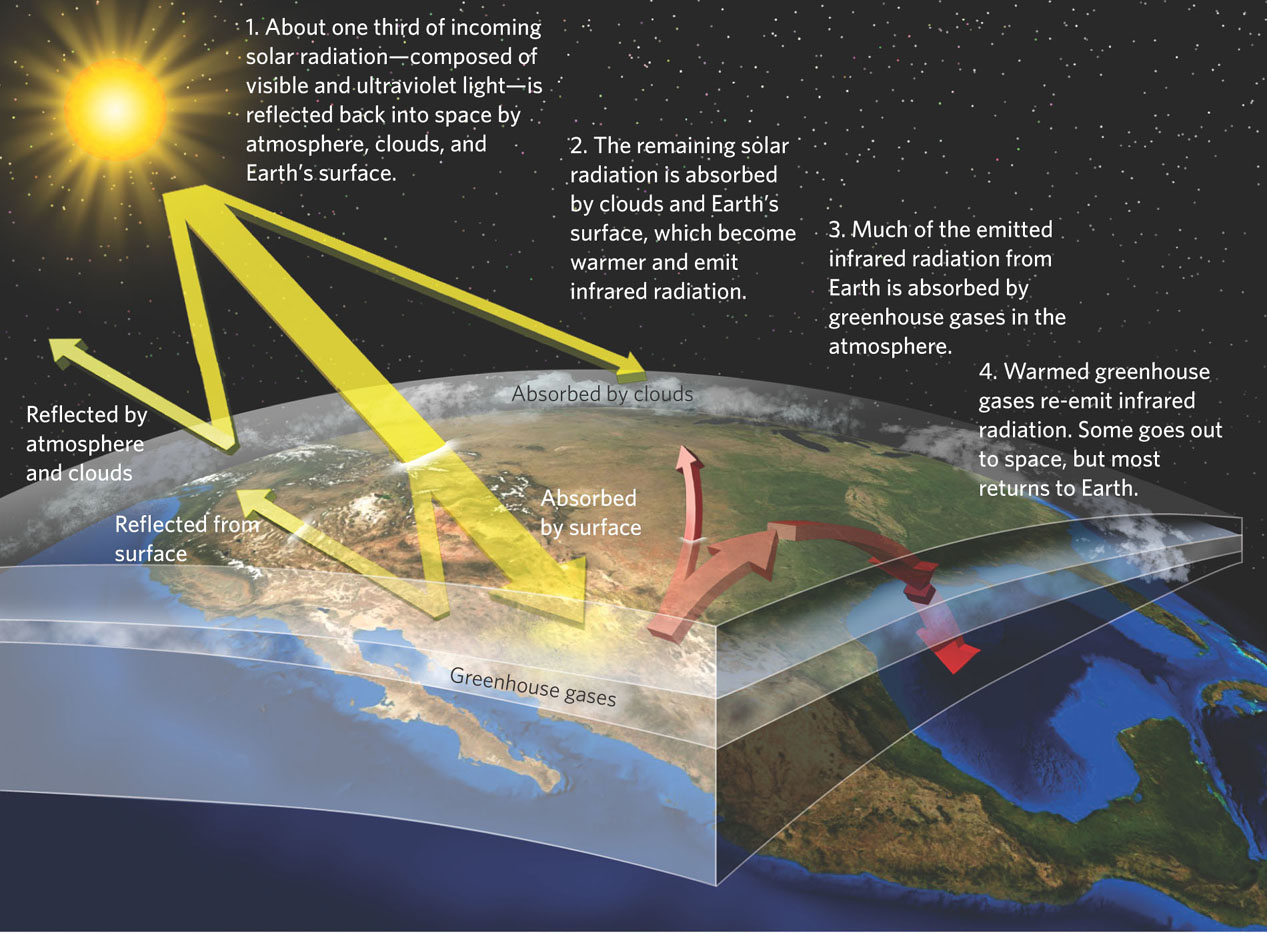
115
Greenhouse effect The process of solar radiation striking Earth, being converted to infrared radiation and being absorbed and re-emitted by atmospheric gases.
The solar radiation that we call visible light easily passes through the gases of the atmosphere. Infrared radiation, however, is readily absorbed by gases in the atmosphere. The gases are warmed by the infrared radiation and then re-emit infrared radiation in all directions. Some of this energy goes out into space and some goes back toward the planet’s surface. This process of solar radiation striking Earth, being converted to infrared radiation, and then being absorbed and reradiated by atmospheric gases is known as the greenhouse effect. The name comes from the fact that the effect resembles a gardener’s greenhouse with windows that hold in the heat from solar radiation.
Greenhouse Gases
There are many different gases in the atmosphere, but only those that absorb and re-emit infrared radiation and contribute to the greenhouse effect are known as greenhouse gases.In fact, if we exclude water vapor, 99 percent of the gases in the atmosphere are oxygen (O2) and nitrogen (N2) and neither gas functions as a greenhouse gas. This means that greenhouse gases, which play such a major role in keeping our planet warm, compose only a small fraction of the atmosphere. The two most prevalent greenhouse gases are water vapor (H2O) and carbon dioxide (CO2). Other naturally occurring greenhouse gases include methane (CH4), nitrous oxide (N2O), and ozone (O3). These gases have a variety of natural sources: water vapor comes from large bodies of water and the transpiration of plants; CO2 comes from decomposition, respiration of organisms, and volcanic eruptions; N2O comes from wet soils and low-oxygen regions of water bodies; and CH4 comes from anaerobic decomposition. The naturally occurring greenhouse effect is quite beneficial to organisms on Earth. Without this phenomenon, the average temperature on Earth, which is currently 14°C, would be a much colder –18°C.
116
The concentrations of greenhouse gases in the atmosphere are increasing. As we noted in Chapter 4, the concentration of CO2 in the atmosphere has substantially increased over the past 2 centuries due to increased combustion of fossil fuels by automobiles, electric generating plants, and other industrial processes. At the same time, there have also been increases in methane and nitrous oxide from a variety of anthropogenic sources that include agriculture, landfills, and the combustion of fossil fuels. Finally, there are gases that are not naturally produced, such as chlorofluorocarbons that have been manufactured to serve as propellants in aerosol cans and as refrigerants in freezers, refrigerators, and air conditioners. Although these human-created compounds exist at much lower concentrations than water vapor or CO2, each molecule can absorb much more infrared radiation than H2O and CO2, and persists in the atmosphere for hundreds of years. A steady increase of these manufactured gases over the past 2 centuries has raised concerns among scientists, environmentalists, and policy makers.
Because greenhouse gases absorb and re-emit infrared radiation to Earth and its atmosphere, it makes sense that an increase in the concentration of greenhouse gases in the atmosphere would cause an increase in the average temperature of Earth. This expectation has been borne out. Based on thousands of measurements made throughout the world, the average air temperature of the planet’s surface increased by approximately 1°C from 1880 to 2011. While some regions, such as Antarctica, have become 1°C to 2°C cooler, other regions, such as northern Canada, have become up to 4°C warmer. In fact, over the 131-year period of monitoring temperatures around the world, 9 of the 10 warmest years occurred from 2000 to 2011. As we will see at the end of this chapter, these temperature changes are altering global climates.