Reproduction can be sexual or asexual
All organisms reproduce but they have a variety of ways in which they accomplish this task. In plants, animals, fungi, and protists, reproduction may be accomplished through either sexual reproduction or asexual reproduction. In this section, we will look at the two processes and compare their costs and benefits.
Sexual Reproduction
Sexual reproduction A reproduction mechanism in which progeny inherit DNA from two parents.
Gonads The primary sexual organs in animals.
As we discussed in Chapter 7, reproductive function in most animals and plants is divided between two sexes. When progeny inherit DNA from two parents, we say they are the result of sexual reproduction.
Sexual gametes are produced through meiosis within the primary sexual organs, known in animals as gonads. Meiosis produces haploid cells, each of which contains a single full set of chromosomes. In animals, these haploid cells can immediately act as gametes. In plants and many protists, the haploid cells develop into multicellular, haploid stages of the life cycle that eventually produce gametes. Of the two sets of chromosomes inherited by an organism’s parents, the distribution of the chromosomes into the haploid cells is generally random and the mixing of chromosomes from the two parents results in new combinations of genes in the offspring. Ultimately, two gametes join together in an act of fertilization to produce a diploid zygote.
Asexual Reproduction
Asexual reproduction A reproduction mechanism in which progeny inherit DNA from a single parent.
In contrast to sexual reproduction, progeny produced by asexual reproduction inherit DNA from a single parent. This can be accomplished through either vegetative reproduction or parthenogenesis.
Vegetative Reproduction
Vegetative reproduction A form of asexual reproduction in which an individual is produced from the nonsexual tissues of a parent.
Vegetative reproduction occurs when an individual is produced from the nonsexual tissues of a parent. Many plants can reproduce by developing new shoots that sprout from leaves, roots, or rhizomes (i.e., underground shoots). Figure 9.1 shows an example of this in a walking fern (Asplenium rhizophyllum), which produces offspring when the tips of its leaves touch the soil. If you have ever placed a plant clipping into a glass of water and watched it grow roots to become a new plant, you have witnessed this type of reproduction. Individuals that descend asexually from the same parent and bear the same genotype are known as clones. Many simple animals, such as hydras, corals, and their relatives, also reproduce in this way; they produce buds along their body that develop into new individuals. Bacteria and some species of protists reproduce by duplicating their genes and then dividing the cell into two identical cells, a process known as binary fission.

Clones Individuals that descend asexually from the same parent and bear the same genotype.
Binary fission Reproduction through duplication of genes followed by division of the cell into two identical cells.
209
Parthenogenesis
Parthenogenesis A form of asexual reproduction in which an embryo is produced without fertilization.
In contrast to vegetative reproduction, some organisms reproduce asexually by producing an embryo without fertilization, a process known as parthenogenesis. In most cases, parthenogenetically produced offspring arise from diploid eggs, which do not require any genetic contribution from sperm. Parthenogenesis has evolved in plants and several groups of invertebrates including water fleas, aphids, and the Cape honeybees mentioned at the beginning of this chapter. Animal species that reproduce only by parthenogenesis are typically composed entirely of females.
Parthenogenesis is relatively rare in vertebrates. It has never been observed as a natural occurrence in mammals but has been confirmed in a few species of lizards, amphibians, birds, and fishes. For a long time it was thought that snakes and sharks were not capable of parthenogenesis. In 2007, however, researchers confirmed that a virgin female hammerhead shark (Sphyrna tiburo) gave birth to daughters that were genetically identical to the mother. In 2011, researchers discovered that a female boa constrictor gave birth to two litters of daughters through parthenogenesis (Figure 9.2). The growing evidence suggests that parthenogenesis may be more common than we currently appreciate and that some species can reproduce through both sexual reproduction and parthenogenesis.

210
Parthenogenesis can produce offspring that are clones of the parent or offspring that are genetically variable. Clones are produced when germ cells develop directly into egg cells without going through meiosis. In contrast, genetically variable offspring are produced when germ cells proceed partially or entirely through meiosis. In partial meiosis, germ cells pass through the first meiotic division, but suppression of the second meiotic division results in diploid egg cells. Although a sexual union is not involved, these eggs differ from one another genetically because of recombination between pairs of homologous chromosomes and the independent assortment of chromosomes during the first meiotic division. When the germ cells experience complete meiosis, the gamete-forming cells of the female are haploid and then fuse to form a diploid embryo.
Costs of Sexual Reproduction
Sexual and asexual reproduction are both viable strategies, but sexual reproduction comes with a number of costs. For example, sexual organs require considerable energy and use resources that could be devoted to other purposes. In addition, mating itself can be a substantial task. Many plants must produce floral displays to attract pollinators and most animals conduct elaborate courtship rituals to attract mates. These activities require time and resources. They can also elevate the risk of herbivory, predation, and parasitism.
For organisms in which the sexes are separate—that is, in which an individual is either male or female—sexual reproduction has an additional cost of reduced fitness. To understand this cost, we need to remember that every parent’s goal is to leave as many copies of its genes as possible in the next generation; this maximizes the parent’s fitness. In the case of asexual reproduction, as shown in Figure 9.3a, a parent contributes two sets of chromosomes to each of its offspring. In the case of sexual reproduction, each parent contributes only one set of chromosomes to each offspring because the gametes produced by meiosis are haploid, as shown in Figure 9.3b. Females using either mode of reproduction produce the same number of offspring, but the female parent using sexual reproduction leaves behind half as many copies of its genes as a female using asexual reproduction. This 50 percent reduction due to sexual versus asexual reproduction is known as the cost of meiosis.

Cost of meiosis The 50 percent reduction in the number of a parent’s genes passed on to the next generation via sexual reproduction versus asexual reproduction.
The cost of meiosis can be counterbalanced by hermaphroditism, a reproductive strategy that is found in most plants and in many invertebrates. Consider an individual that uses sexual reproduction and possesses both male and female function. Such an individual can contribute one set of its genes to offspring produced through female function and another set to offspring produced through male function. As shown in Figure 9.3c, this allows a hermaphrodite to contribute twice as many copies of its genes to its offspring than is possible for an individual that can be only male or female.
211
The cost of meiosis can also be offset when the sexes are separate and the male helps the female take care of the offspring. Consider the case in which a male’s parental assistance allows the female to raise twice as many offspring as she could care for alone. In this case, then the 50 percent cost of meiosis is offset by doubling the number of offspring that can be raised when both parents care for them.
Benefits of Sexual Reproduction
If sexual reproduction is so costly, then it must persist because it provides substantial benefits. These benefits include purging harmful mutations and creating genetic variation that helps offspring deal with future environmental variation, including the existence of rapidly evolving parasites and pathogens.
Purging Mutations
Mutations occur in all organisms, and most are harmful. In asexually reproducing organisms there is no way to lose mutations from one generation to the next, so mutations continue to accumulate over generations, especially if the asexual parents produce clonal offspring. In contrast, sexually reproducing organisms can lose deleterious mutations during meiosis, which involves the random assortment of genes, or after fertilization of the gametes. Of all the gametes that are produced, those that form zygotes may not contain the mutation. Alternatively, if some of the gametes used to make offspring do contain the mutation, the union of two gametes that both contain the recessive mutation produces offspring that are homozygous recessive for the harmful mutation. A homozygous recessive offspring will express the harmful mutation and, as a result, it is likely that the offspring will not be viable and will fail to pass the mutation to the next generation.
Because species that only reproduce asexually do not have any means of purging mutations, deleterious mutations slowly accumulate over many generations. In time we would expect individuals of such a species to experience poor growth, survival, and reproduction, which would lead to eventual extinction. If this hypothesis is correct, then species that use asexual reproduction today would have adopted this mode of reproduction only recently. Asexually reproducing species that arose long ago would probably have gone extinct by now.
To test the hypothesis that asexual reproducing species do not persist in nature as long as sexual reproducing species, we can look at the patterns of asexual reproduction within a phylogeny. If the hypothesis is correct, we should observe that asexual reproduction has evolved relatively recently. For example, most vertebrate species that reproduce asexually belong to genera that have a sexual ancestor and contain mostly sexual species, with asexual species that are relatively recently evolved. We observe this pattern with salamanders in the genus Ambystoma, fishes in the genus Poeciliopsis, and lizards in the genus Cnemidophorus. This observation suggests that purely asexual species typically do not have long evolutionary histories. If they did, we would expect to see large groups of related species—such as most species within a genus—all using asexual reproduction. Such a pattern would suggest that their common ancestor used asexual reproduction. In fact, the long-term evolutionary persistence of asexual populations appears to be low. This fits with the explanation that accumulation of mutations and lack of genetic variation cause species that reproduce asexually to go extinct.
However, not all asexual species fit this pattern. For example in the bdelloid rotifers, an ancient group of more than 300 species of freshwater and soil organisms, all species are asexual and entirely female. Similarly, some groups of protists have existed for hundreds of millions of years and appear to lack sexual reproduction. One way that such species could avoid extinction is by producing offspring more rapidly than new deleterious mutations arise, so that some individuals would always retain the nonmutated parental genotype and produce the next generation, a process known as clonal selection. However, groups such as these continue to challenge our efforts to understand the full range of costs and benefits that favor the evolution of sexual or asexual reproduction.
Genetic Variation and Future Environmental Variation
A second benefit of sexual reproduction is the production of offspring with greater genetic variation. If the environment were homogeneous across time and space, parents that are well adapted to the environment could use asexual reproduction to produce clonal offspring that are also well adapted. However, as we have discussed in earlier chapters, environmental conditions typically change across time and space. As a result, offspring are likely to encounter different environmental conditions than their parents did. Because environmental conditions vary, offspring with genetic variation have an increased probability of possessing gene combinations that will help them adapt to different environmental conditions. Most theoretical models that examine the importance of abiotic environmental variation conclude that temporal and spatial variation in the physical environment does not produce a large enough advantage to offset the cost of meiosis. A promising alternative explanation is that temporal and spatial variation in the biotic environment— particularly variation in pathogens—provides a large advantage to sexual reproduction.
212
Genetic Variation and Evolving Parasites and Pathogens
Red Queen hypothesis The hypothesis that sexual selection allows hosts to evolve at a rate that can counter the rapid evolution of parasites.
To understand why sexual reproduction provides an evolutionary benefit when species experience variation in pathogens, we first must realize that pathogens have much shorter generation times and much larger population sizes than most of the host species they infect. Because pathogens have the potential to evolve at a much faster rate than their hosts, they can evolve ways to get past host defenses. Without rapid host evolution, pathogens could drive their hosts to low numbers or even to extinction. For example, in 1998 researchers described a newly discovered species of pathogen that was causing widespread amphibian deaths in Central America. The pathogen is a type of chytrid fungus (Batrachochytrium dendrobatidis) that can infect a wide variety of amphibian species. By 2012, the fungus had been detected on every continent inhabited by amphibians. In some parts of the world, including Central America, it appears that this deadly pathogen may have been recently introduced and that many species of frogs in the region have no adaptations to combat it. As a result, scientists now believe that dozens of species have gone extinct.
The harmful effects of pathogens place a premium on hosts to evolve new defenses rapidly. As we have seen, sexual reproduction produces offspring with a greater range of genetic combinations, and some of these combinations might be better suited to combat the pathogen. In short, there is an evolutionary race between hosts, which are trying to evolve adaptations rapidly enough to combat the pathogen, and pathogens, which are trying to evolve adaptations rapidly enough to elude host defenses. The hypothesis that sexual selection allows hosts to evolve at a rate sufficient to counter the rapid evolution of parasites is called the Red Queen hypothesis, after the famous passage in Lewis Carroll’s Through the Looking-Glass, and What Alice Found There, in which the Red Queen tells Alice, “Now, here, you see, it takes all the running you can do, to keep in the same place.”
Testing the Red Queen Hypothesis
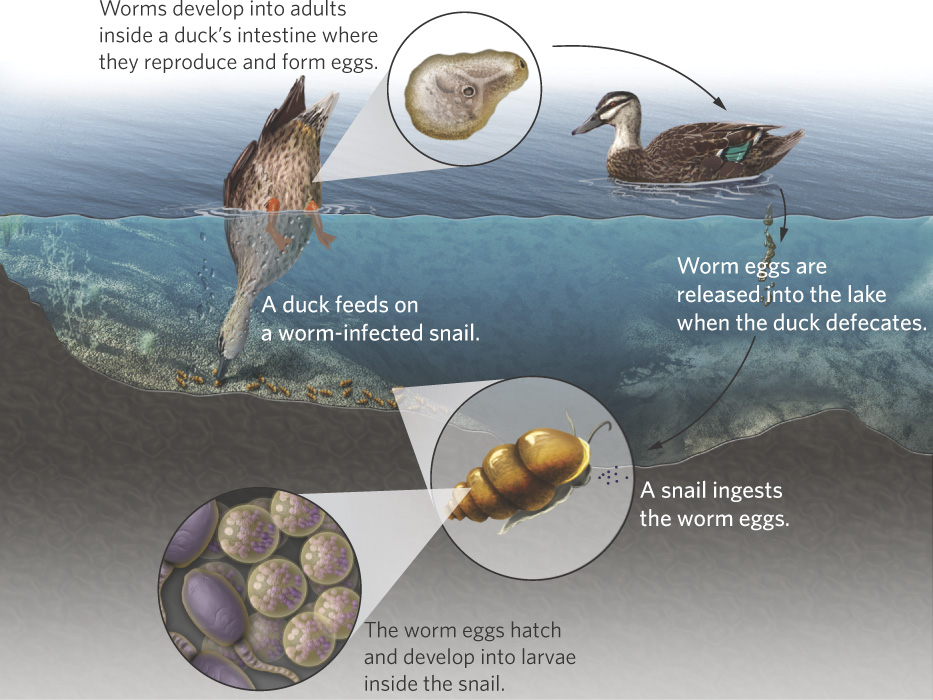
One of the most compelling tests of the Red Queen hypothesis focuses on a species of freshwater snail (Potamopyrgus antipodarum) that is a common inhabitant of lakes and streams in New Zealand. The snails can become infected by parasites, including trematode worms in the genus Microphallus. The life cycle of the pathogen is shown in Figure 9.4. The worm’s life cycle begins when the snails ingest worm eggs. The eggs hatch into larvae that form cysts in the sexual organs and cause the snails to become sterile. Ducks then eat the infected snails and the pathogens mature sexually inside the intestines of the ducks, where they produce eggs asexually. These eggs exit the duck when it defecates in the water, thereby completing the cycle. Not surprisingly, Microphallus is most abundant in shallow waters of lakes where ducks feed.
The snail’s mode of reproduction depends on the depth of water in which it lives. In the shallower regions of the lake, where the parasitic worm is more common, a higher proportion of the snails use sexual reproduction. Such populations contain about 13 percent males—enough to maintain some genetic diversity through sexual reproduction. In deeper regions, where the parasite is rare, a higher proportion of the snails use asexual reproduction. Although asexual populations reproduce faster than sexual populations, asexual clones cannot persist in the face of high rates of parasitism. As a result, the asexual snails do not survive well in the shallower regions of the lake where they are more likely to encounter the parasitic worm.
If the parasitic worms evolve to specialize on the snails with which they coexist, then parasites living in the shallow water should be good at infecting snail populations that live in shallow water. Similarly, other parasites that live in deep water should be good at infecting snail populations in that region. Researchers tested this hypothesis with parasites and snails from several different lakes in New Zealand. As shown in Figure 9.5, shallow-water snails from several different lakes were infected most readily by shallow-water parasites, and deep-water snails were infected most readily by deep-water parasites. Averaged across all parasite sources, infection rates were relatively low in deep-water snails because few parasites live in this region and, therefore, have had less opportunity to evolve an ability to infect the deep-water snails. However, because deep-water habitats contain fewer parasites, asexual lineages of snails have a reproductive advantage over sexual lineages because of their more rapid reproduction.

Recent studies have continued to support the Red Queen hypothesis. In the roundworm (Caenorhabditis elegans), for example, researchers raised individuals in the laboratory that were genetically destined to reproduce either sexually or asexually and then exposed populations containing two different types of worms to a bacterial parasite. In 2011, the researchers reported that when they allowed the bacteria to evolve to infect the worms, the parasite quickly drove the asexual worms to extinction. In contrast, the sexual worms continually evolved resistance against the parasite and persisted. When the researchers prevented the bacteria from evolving, the asexual individuals came to dominate the population.
213