THE MULTI-HIT MODEL
Inheriting certain BRCA alleles dramatically increases the risk of cancer, but it doesn’t mean that the disease will necessarily develop. In most cases, hereditary cancer occurs only when additional, nonhereditary mutations in a cell accumulate. Similarly, the accumulation of harmful somatic mutations in a cell can lead to cancer, even in someone with no genetic predisposition. This is known as the multi-hit model of cancer, where each “hit” is a mutation, and multiple hits are needed to cause the disease.
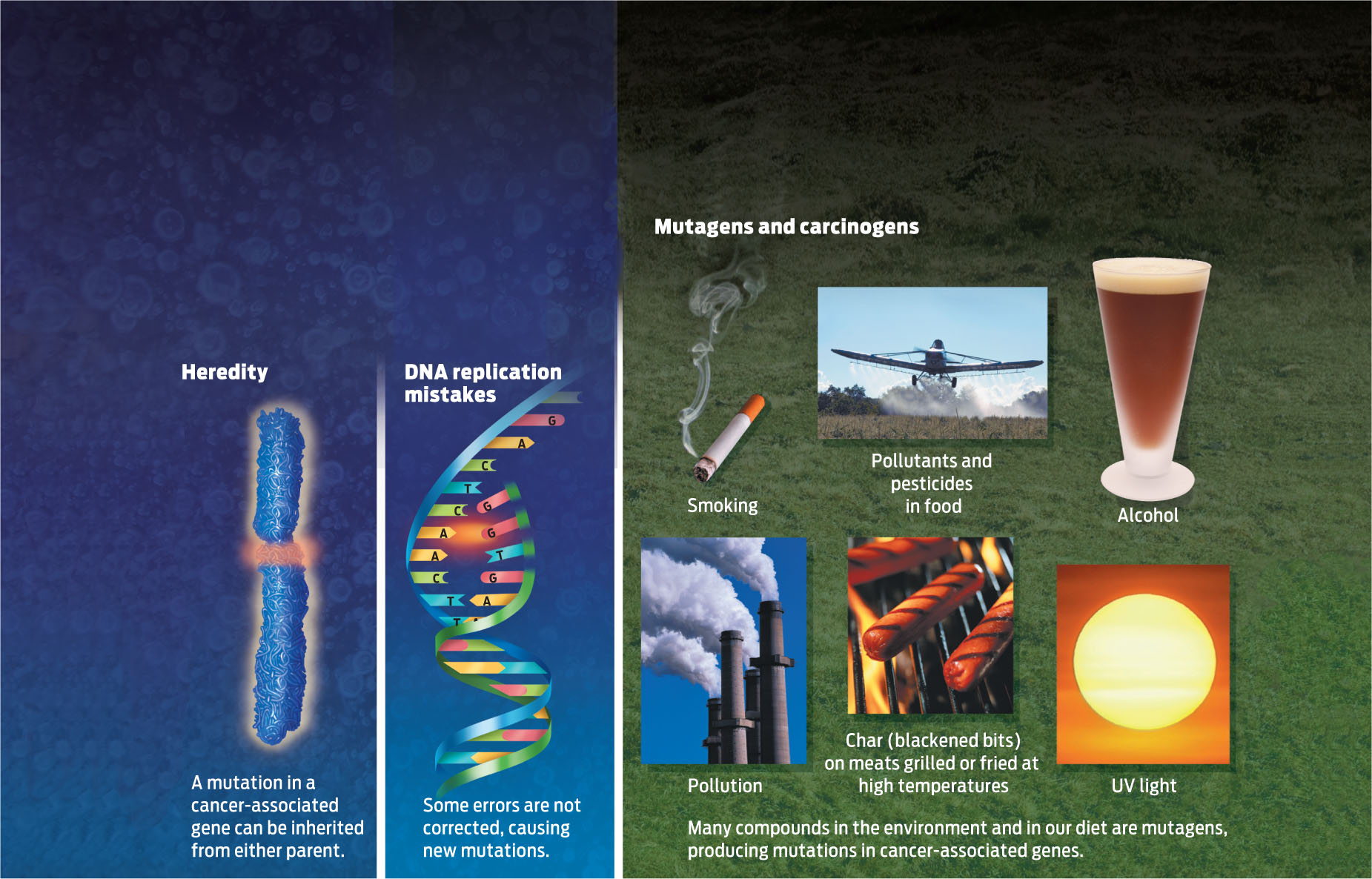
What causes mutations, other than DNA replication mistakes? Environmental insults such as chemicals, ultraviolet light, radiation, and other factors can damage DNA and cause it to mutate. Exposure to ultraviolet light, for example, damages the DNA in our skin cells and can lead to skin cancer.
Environmental insults such as chemicals, ultraviolet light, radiation, and other factors can damage DNA and cause it to mutate.
MUTAGEN Any chemical or physical agent that can damage DNA by changing its nucleotide sequence.
220
CARCINOGEN Any chemical agent that causes cancer by damaging DNA. Carcinogens are a type of mutagen.
Physical or chemical agents that cause mutations are called mutagens. Chemicals and other factors such as pesticides and pollutants that can cause cancer are a class of mutagen known as carcinogens because they damage DNA in a way that can lead to cancer. Not all mutagens originate outside the body. Some of the reactions that occur in the mitochondria during cellular respiration (see Chapter 6), for example, produce DNA-damaging molecules known as free radicals (INFOGRAPHIC 10.5) .
When genes become mutated, their DNA sequence is altered. There are several ways that DNA mutations can arise: they may be inherited; they may occur during DNA replication if errors are not corrected; they can also arise from environmental insults.
PROTO-ONCOGENE A gene that codes for a protein that helps cells divide normally.
Normally cells are able to repair such DNA damage. But very rarely a mistake remains uncorrected, and over time and with age, if enough mutations accumulate in the same cell, that cell may begin to divide abnormally and become cancerous. Such acquired somatic mutations can develop throughout a person’s life as he or she is exposed to carcinogenic environmental insults and as cells divide.
TUMOR SUPPRESSOR GENE A gene that codes for proteins that monitor and check cell cycle progression. When these genes mutate, tumor suppressor proteins lose normal function.
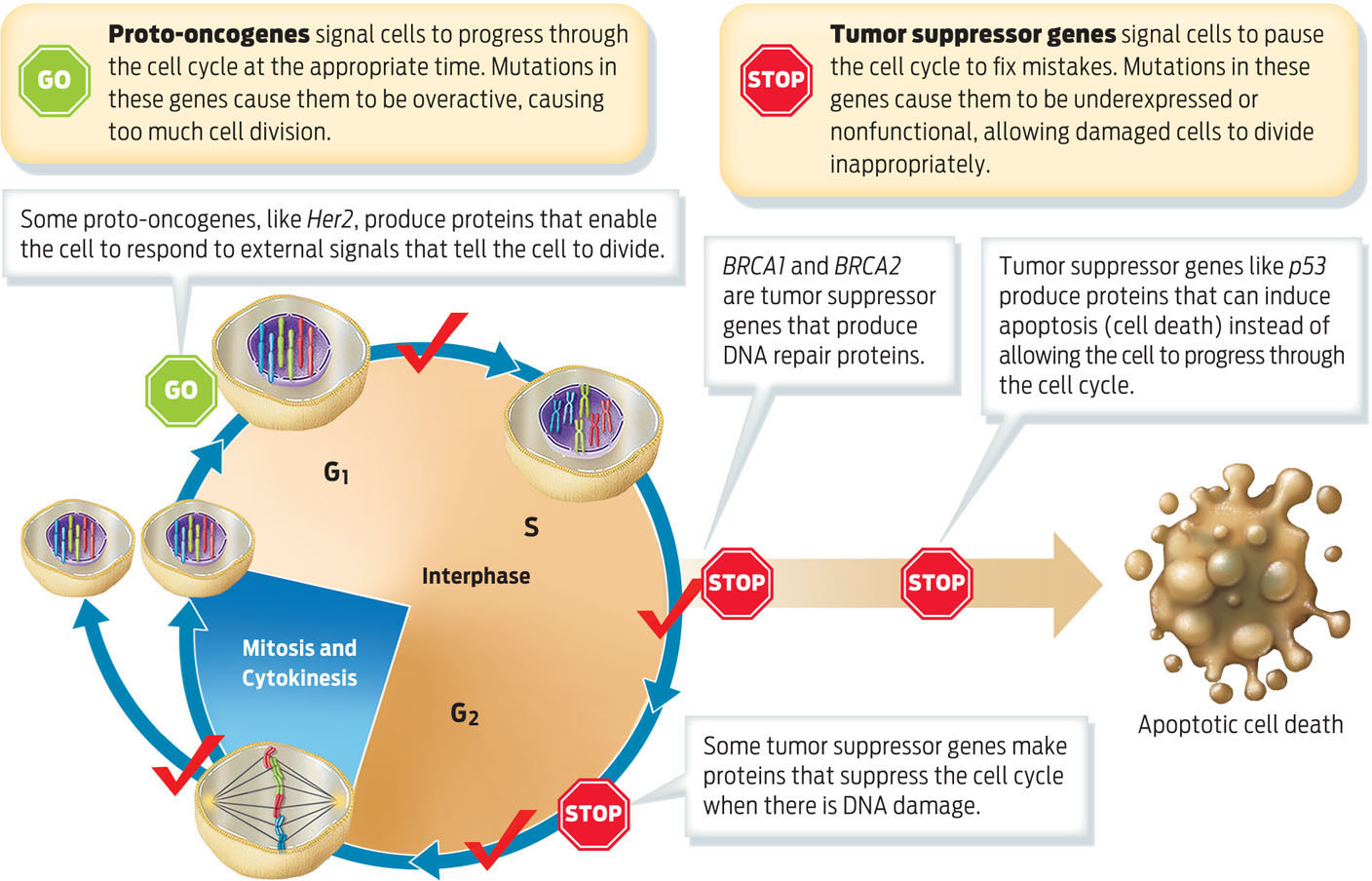
Mutations that cause cancer typically occur in two categories of genes that influence the cell cycle: proto-oncogenes and tumor suppressor genes. Normal proto-oncogenes promote cell division and cell differentiation, but only in response to appropriate signals. When proto-oncogenes are mutated, they can become permanently “turned on,” or activated, stimulating cells to divide all the time. In this state they are called oncogenes–genes that cause cancer. In other words, oncogenes are proto-oncogenes that have mutated to become overexpressed or permanently activated. Her2, a gene overexpressed in certain types of breast cancer, is an example of a proto-oncogene.
ONCOGENE A mutated and overactive form of a proto-oncogene. Oncogenes drive cells to divide continually.
Tumor suppressor genes, also known as tumor suppressors, normally pause cell division, repair damaged DNA, and initiate apoptosis, or cell death. Tumor suppressor genes cause cancer when they are inactivated by mutation. “You can think of the suppressors as brakes and the oncogenes as the accelerators,” Sellers of the H. Lee Moffitt Cancer Center and Research Institute explains. “They are sort of the yin and yang of each other.” If a proto-oncogene is mutated, it’s as if the accelerator is stuck down and the car–cell division–keeps going; if a tumor suppressor gene is mutated, it’s as if the brakes don’t work and the car cannot be stopped. Both BRCA1 and BRCA2 are tumor suppressors that, when normally expressed, code for proteins that help the cell progress normally through the cell cycle and respond to DNA damage (INFOGRAPHIC 10.6) .
During the cell cycle, proteins regulate whether the cell is ready to continue to the next stage or if the cell requires additional time to repair DNA damage before progressing. The proteins that regulate these checkpoints are made by proto-oncogenes and tumor suppressor genes. Accumulated mutations in these types of genes cause cancer.
221
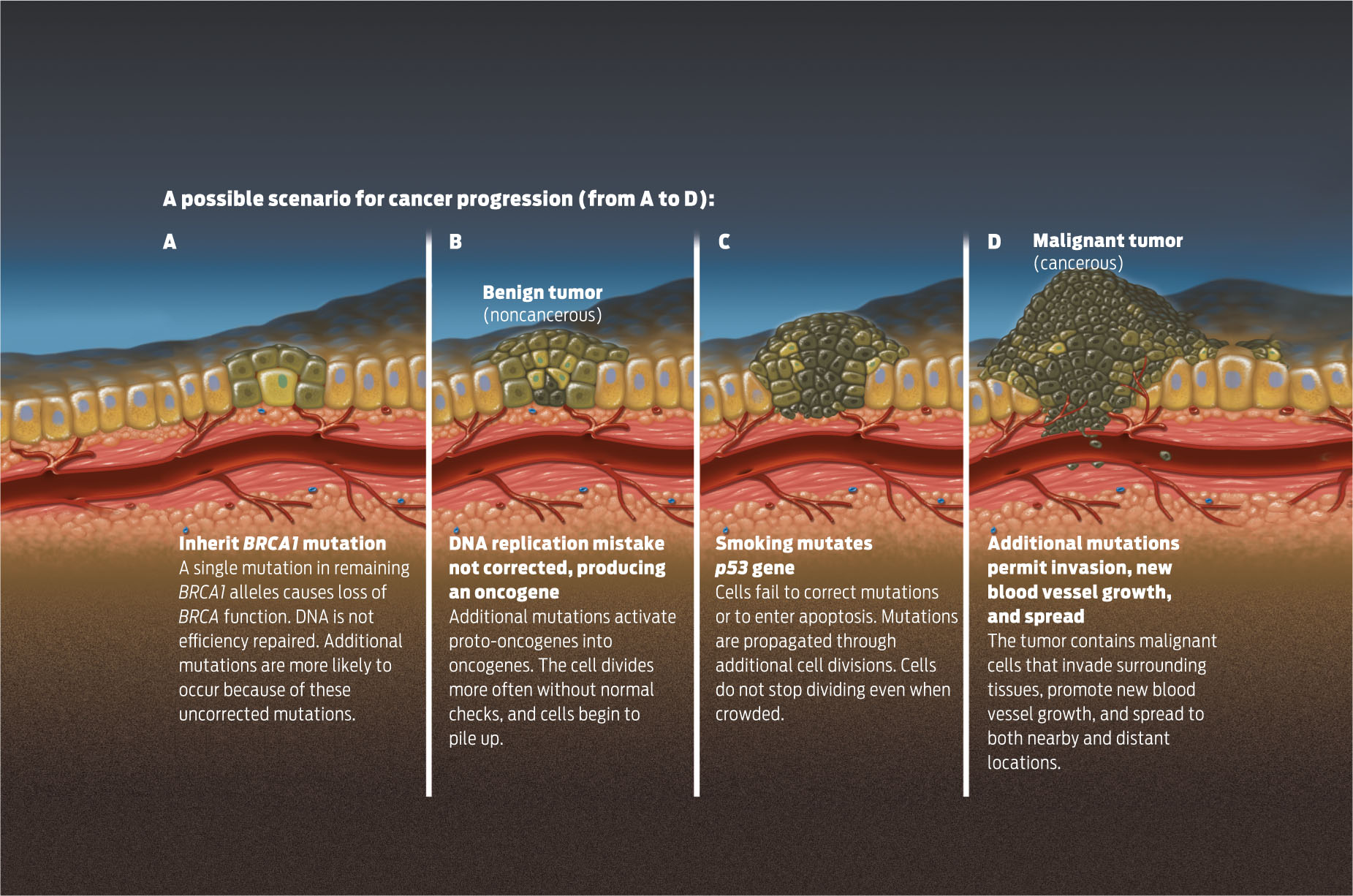
It usually takes more than a single mutation in a cell to cause cancer. In most cases, a cell will become cancerous only after it has acquired mutations in several genes that regulate the cell cycle or repair DNA damage. The collective mutated genes can include a combination of tumor suppressor genes that have lost their function and proto-oncogenes that have been activated to oncogenes. This is one reason why cancer affects people more as they age: as cells accumulate mutations over time through exposure to carcinogens and repeated rounds of cell division, the chances increase that a cell will accumulate enough mutations to become cancerous.
BENIGN TUMOR A noncancerous tumor that will not spread throughout the body.
222
MALIGNANT TUMOR A cancerous tumor that spreads throughout the body.
In the multi-hit model, tumors develop in stages following mutations. After one or two hits, a benign tumor may form. After several more, a malignant tumor may result. Generally, the word “cancer” applies to malignant tumors, which have the capacity to invade other tissues and spread to other parts of the body, or metastasize (INFOGRAPHIC 10.7) .
It takes more than a single mutation to cause cancer. Individuals who have inherited high-risk mutations require fewer additional mutations to get to cancer, and therefore develop cancer at a much earlier age.
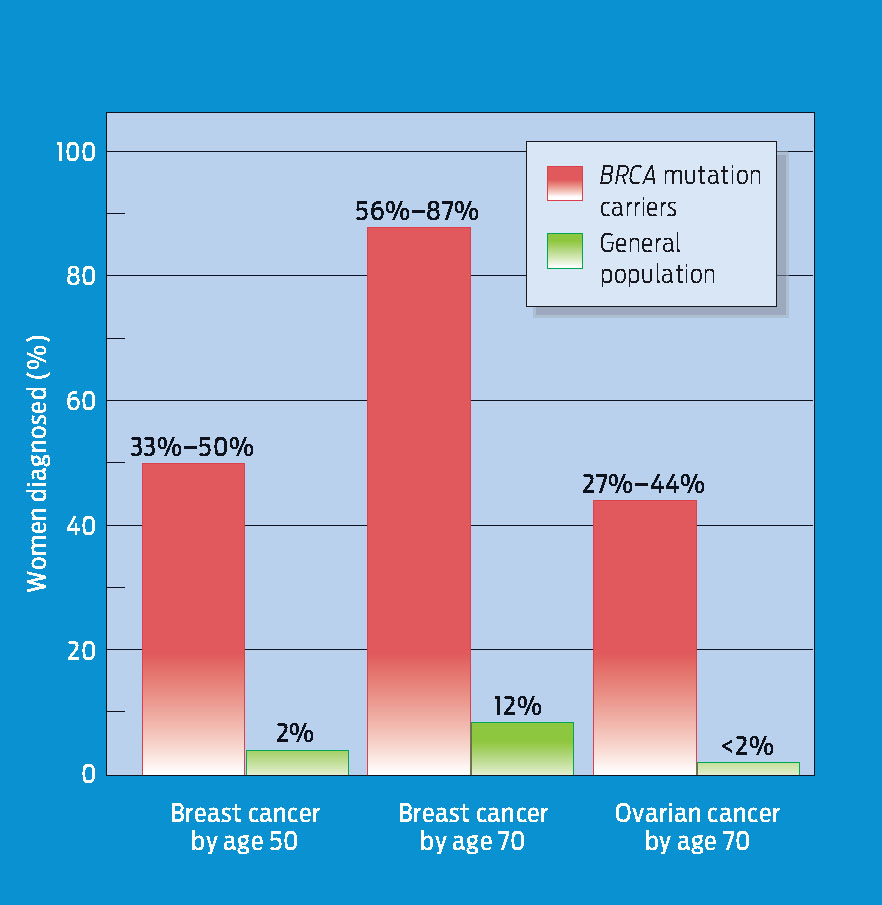
People who have inherited high-risk mutations start life with at least one cancer-predisposing mutation, so they require fewer additional mutations to develop cancer. For example, Ahern was born with a predisposing mutation in one of her BRCA1 alleles. If a second mutation in one of her somatic cells disables her second BRCA1 allele, that cell and all its descendants will no longer be able to respond effectively to repair DNA damage. Consequently, the cells of women with BRCA mutations accumulate DNA damage at a faster rate, which is why hereditary breast cancer often strikes women who are in their 30s and 40s-much younger than women who have no inherited predisposition to cancer (INFOGRAPHIC 10.8) .
Women with one copy of particular BRCA1 alleles (“carriers”) are at higher risk of developing breast cancer at earlier ages.
223
Since BRCA genes are expressed in many cell types in addition to breast tissue, mutations in either gene raise the risk of cancers in other organs, too. Scientists have linked mutations in both genes to a higher than average risk of prostate, colon, and pancreatic cancers, among others. But the breasts and ovaries are at especially high risk of developing cancers because they respond to the hormone estrogen, which causes cells in these organs to divide more often. In breast tissue, for example, the rise in estrogen during a woman’s menstrual cycle signals cells lining the milk glands to divide to prepare to produce milk should a woman become pregnant.
The BRCA genes aren’t the only ones that predispose women to breast cancer. Scientists now think that genes other than BRCA cause up to half of all hereditary breast cancers. Other inherited mutations in tumor suppressor genes and proto-oncogenes have been linked to various other cancers as well.